Autocatalytic reaction
Encyclopedia
Autocatalytic reactions are chemical reaction
s in which at least one of the reactants is also a product
. The rate equation
s for autocatalytic reactions are fundamentally nonlinear. This nonlinearity can lead to the spontaneous generation of order. A dramatic example of this order is that which is found in living systems. This spontaneous order creation seems to contradict the Second Law of Thermodynamics
. This contradiction is resolved when the disorder of both the system and its surroundings are taken into account.
states that the disorder (entropy
) of a physical or chemical system and its surroundings (a closed system
) must increase with time. In other words, systems left to themselves must become increasingly random. To say it yet another way, orderly energy of a system like uniform motion must degrade eventually to the random motion of particles in a heat bath.
This seems to run counter to experience. There are many instances in which physical systems spontaneously become emergent
or orderly. For example, despite the destruction they do, hurricanes have a very orderly vortex
motion when compared with the random motion of the air molecules in a closed room. Even more spectacular is the order created by chemical systems; the most dramatic being the order associated with life.
Our experience is consistent with the Second Law. The Second Law states that the total disorder of a system and its surroundings must increase with time. Order can be created in a system by an even greater decrease in order of the systems surroundings. In the hurricane example, hurricanes are formed from unequal heating within the atmosphere. The Earth's atmosphere is then far from thermal equilibrium
. The order of the Earth's atmosphere increases, but at the expense of the order of the sun. The sun is becoming more disorderly as it ages and throws off light and material to the rest of the universe. The total disorder of the sun and the earth increases despite the fact that orderly hurricanes are generated on earth.
A similar example exists for living chemical systems. The sun provides energy to green plants. The green plants are food for other living chemical systems. The energy absorbed by plants and converted into chemical energy generates a system on earth that is orderly and far from chemical equilibrium
. Here, the difference from chemical equilibrium is determined by an excess of reactants over the equilibrium amount. Once again, order on earth is generated at the expense of entropy increase of the sun. The total entropy of the earth and the rest of the universe increases, consistent with the Second Law.
Not all chemical reactions, however, generate order. The class of reactions most closely associated with order creation is the class of autocatalytic reactions
. These are reactions in which one or more of the products are the same as one or more of the reactants. Simple autocatalytic reactions (clock reactions) are known to oscillate in time, thus creating temporal order. Other simple reactions can generate spatial separation of chemical species
generating spatial order. More complex reactions are involved in metabolic pathway
s and metabolic network
s in biological systems.
The transition to order as the distance from equilibrium increases is not usually continuous. Order typically appears abruptly. The threshold between the disorder of chemical equilibrium and order is known as a phase transition
. The conditions for a phase transition can be determined with the mathematical machinery of non-equilibrium thermodynamics
.
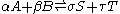
where the Greek letters are stoichiometric coefficients and the capital Latin letters represent chemical species. The chemical reaction proceeds in both the forward and reverse direction. This equation is easily generalized to any number of reactants, products, and reactions.
the forward and reverse reaction rate
s are such that each chemical species is being created at the same rate it is being destroyed. In other words, the rate of the forward reaction is equal to the rate of the reverse reaction.
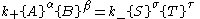
Here, the curly brackets indicate the amount of the chemical species, in moles
, and k+ and k− are rate constants.
 molecules of A are destroyed. For every reverse reaction
molecules of A are destroyed. For every reverse reaction  molecules of A are created. The change in number of moles of A is then
molecules of A are created. The change in number of moles of A is then
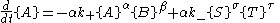
and similarly for the other reactant and products.
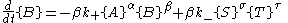
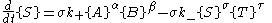
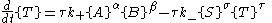
This system of equations has a single stable fixed point
when the forward rates and the reverse rates are equal. This means that the system evolves to the equilibrium state, and this is the only state to which it evolves.
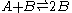
with the rate equations
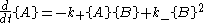
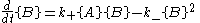 .
.
This reaction is one in which a molecule of species A interacts with a molecule of species B. The A molecule is converted into a B molecule. The final product consists of the original B molecule plus the B molecule created in the reaction.
The key feature of these rate equations is that they are nonlinear; the second term on the right varies as the square of the concentration of B. This feature can lead to multiple fixed points of the system, much like a quadratic equation
can have multiple roots. Multiple fixed points allow for multiple states of the system. A system existing in multiple macroscopic
states is more orderly (has lower entropy) than a system in a single state.
 Consider a coupled set of two autocatalytic reactions in which the concentration of one of the reactants A is much larger than its equilibrium value. In this case the forward reaction rate is so much larger than the reverse rates that we can neglect the reverse rates.
Consider a coupled set of two autocatalytic reactions in which the concentration of one of the reactants A is much larger than its equilibrium value. In this case the forward reaction rate is so much larger than the reverse rates that we can neglect the reverse rates.
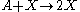
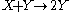
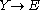
with the rate equations
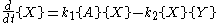
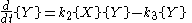 .
.
Here, we have neglected the depletion of the reactant A, since its concentration is so large. The rate constants for the three reactions are ,
, 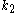 , and
, and  , respectively.
, respectively.
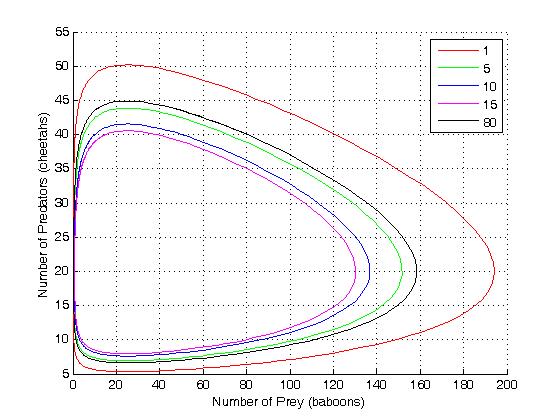
This system of rate equations is known as the Lotka-Volterra equation
and is most closely associated with population dynamics
in predator-prey relationships. This system of equations has an oscillatory behavior. The amplitude of the oscillations depends on the concentration of A. Oscillations of this type are a form of emergent temporal order that is not present in equilibrium.
(see Prigogine reference). It is characterized by the reactions
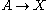
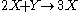
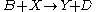
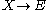
with the rate equations
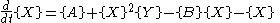
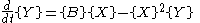
where, for convenience, the rate constants have been set to 1.
The Brusselator has a fixed point at
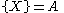
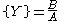 .
.
The fixed point becomes unstable when
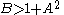
leading to an oscillation of the system. Unlike the Lotka-Volterra equation, the oscillations of the Brusselator do not depend on the amount of reactant present initially. Instead, after sufficient time, the oscillations approach a limit cycle.
(BZ reaction), the Briggs-Rauscher reaction
, the Bray-Liebhafsky reaction
and the iodine clock reaction
. These are oscillatory reactions, and the concentration of products and reactants can be approximated in terms of damped
oscillation
s.
The best-known reaction, the BZ reaction, can be created with a mixture of potassium bromate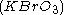 , malonic acid
, malonic acid  , and manganese sulfate
, and manganese sulfate 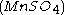 prepared in a heated solution of sulfuric acid
prepared in a heated solution of sulfuric acid 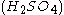 .
.
is the case in which we have two boxes of material separated by a permeable membrane so that material can diffuse
between the two boxes. It is assumed that identical Brusselators are in each box with nearly identical initial conditions. (see Prigogine reference)
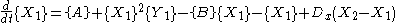
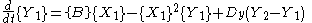
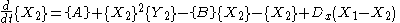
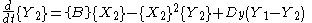
Here, the numerical subscripts indicate which box the material is in. There are additional terms proportional to the diffusion coefficient D that account for the exchange of material between boxes.
If the system is initiated with the same conditions in each box, then a small fluctuation will lead to separation of materials between the two boxes. One box will have a predominance of X, and the other will have a predominance of Y.
, displays temporal order. Glycolysis consists of the degradation of one molecule of glucose and the overall production of two molecules of ATP
. The process is therefore of great importance to the energetics of living cells. The global glycolysis reaction involves glucose
, ADP
, NAD
, pyruvate, ATP
, and NADH.
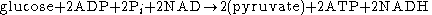 .
.
The details of the process are quite involved, however, a section of the process is autocatalyzed by Phosphofructokinase (PFK). This portion of the process is responsible for oscillations in the pathway that leads to the ability of the process to oscillate between an active and an inactive form. In other words, the autocatalytic reactions can turn the process off and on.
occurs. This abrupt change is known as phase transition
. At the phase transition fluctuations in macroscopic quantities, such as chemical concentrations, increase as the system oscillates between the more ordered state (lower entropy, such as ice water) and the more disordered state (higher entropy, such as liquid water). Also, at the phase transition, macroscopic equations, such as the rate equations, fail. Rate equations can be derived from microscopic considerations. The derivations typically rely on a mean field theory
approximation to microscopic dynamical equations. Mean field theory breaks down in the presence of large fluctuations (see Mean field theory
article for a discussion). Therefore, since large fluctuations occur in the neighborhood of a phase transition, macroscopic equations, such as rate equations, fail. As the initial concentration increases further, the system settles into an ordered state in which fluctuations are again small. (see Prigogine reference)
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s in which at least one of the reactants is also a product
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
. The rate equation
Rate equation
The rate law or rate equation for a chemical reaction is an equation that links the reaction rate with concentrations or pressures of reactants and constant parameters . To determine the rate equation for a particular system one combines the reaction rate with a mass balance for the system...
s for autocatalytic reactions are fundamentally nonlinear. This nonlinearity can lead to the spontaneous generation of order. A dramatic example of this order is that which is found in living systems. This spontaneous order creation seems to contradict the Second Law of Thermodynamics
Second law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
. This contradiction is resolved when the disorder of both the system and its surroundings are taken into account.
Background
The Second Law of ThermodynamicsSecond law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
states that the disorder (entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
) of a physical or chemical system and its surroundings (a closed system
Closed system
-In physics:In thermodynamics, a closed system can exchange energy , but not matter, with its surroundings.In contrast, an isolated system cannot exchange any of heat, work, or matter with the surroundings, while an open system can exchange all of heat, work and matter.For a simple system, with...
) must increase with time. In other words, systems left to themselves must become increasingly random. To say it yet another way, orderly energy of a system like uniform motion must degrade eventually to the random motion of particles in a heat bath.
This seems to run counter to experience. There are many instances in which physical systems spontaneously become emergent
Emergence
In philosophy, systems theory, science, and art, emergence is the way complex systems and patterns arise out of a multiplicity of relatively simple interactions. Emergence is central to the theories of integrative levels and of complex systems....
or orderly. For example, despite the destruction they do, hurricanes have a very orderly vortex
Vortex
A vortex is a spinning, often turbulent,flow of fluid. Any spiral motion with closed streamlines is vortex flow. The motion of the fluid swirling rapidly around a center is called a vortex...
motion when compared with the random motion of the air molecules in a closed room. Even more spectacular is the order created by chemical systems; the most dramatic being the order associated with life.
Our experience is consistent with the Second Law. The Second Law states that the total disorder of a system and its surroundings must increase with time. Order can be created in a system by an even greater decrease in order of the systems surroundings. In the hurricane example, hurricanes are formed from unequal heating within the atmosphere. The Earth's atmosphere is then far from thermal equilibrium
Thermal equilibrium
Thermal equilibrium is a theoretical physical concept, used especially in theoretical texts, that means that all temperatures of interest are unchanging in time and uniform in space...
. The order of the Earth's atmosphere increases, but at the expense of the order of the sun. The sun is becoming more disorderly as it ages and throws off light and material to the rest of the universe. The total disorder of the sun and the earth increases despite the fact that orderly hurricanes are generated on earth.
A similar example exists for living chemical systems. The sun provides energy to green plants. The green plants are food for other living chemical systems. The energy absorbed by plants and converted into chemical energy generates a system on earth that is orderly and far from chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
. Here, the difference from chemical equilibrium is determined by an excess of reactants over the equilibrium amount. Once again, order on earth is generated at the expense of entropy increase of the sun. The total entropy of the earth and the rest of the universe increases, consistent with the Second Law.
Not all chemical reactions, however, generate order. The class of reactions most closely associated with order creation is the class of autocatalytic reactions
Autocatalysis
A single chemical reaction is said to have undergone autocatalysis, or be autocatalytic, if the reaction product itself is the catalyst for that reaction....
. These are reactions in which one or more of the products are the same as one or more of the reactants. Simple autocatalytic reactions (clock reactions) are known to oscillate in time, thus creating temporal order. Other simple reactions can generate spatial separation of chemical species
Chemical species
Chemical species are atoms, molecules, molecular fragments, ions, etc., being subjected to a chemical process or to a measurement. Generally, a chemical species can be defined as an ensemble of chemically identical molecular entities that can explore the same set of molecular energy levels on a...
generating spatial order. More complex reactions are involved in metabolic pathway
Metabolic pathway
In biochemistry, metabolic pathways are series of chemical reactions occurring within a cell. In each pathway, a principal chemical is modified by a series of chemical reactions. Enzymes catalyze these reactions, and often require dietary minerals, vitamins, and other cofactors in order to function...
s and metabolic network
Metabolic network
A metabolic network is the complete set of metabolic and physical processes that determine the physiological and biochemical properties of a cell...
s in biological systems.
The transition to order as the distance from equilibrium increases is not usually continuous. Order typically appears abruptly. The threshold between the disorder of chemical equilibrium and order is known as a phase transition
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
. The conditions for a phase transition can be determined with the mathematical machinery of non-equilibrium thermodynamics
Non-equilibrium thermodynamics
Non-equilibrium thermodynamics is a branch of thermodynamics that deals with systems that are not in thermodynamic equilibrium. Most systems found in nature are not in thermodynamic equilibrium; for they are changing or can be triggered to change over time, and are continuously and discontinuously...
.
Chemical reactions
A chemical reaction of two reactants and two products can be written as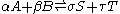
where the Greek letters are stoichiometric coefficients and the capital Latin letters represent chemical species. The chemical reaction proceeds in both the forward and reverse direction. This equation is easily generalized to any number of reactants, products, and reactions.
Chemical equilibrium
In chemical equilibriumChemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
the forward and reverse reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
s are such that each chemical species is being created at the same rate it is being destroyed. In other words, the rate of the forward reaction is equal to the rate of the reverse reaction.
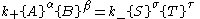
Here, the curly brackets indicate the amount of the chemical species, in moles
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
, and k+ and k− are rate constants.
Far from equilibrium
Far from equilibrium, the forward and reverse reaction rates no longer balance and the concentration of reactants and products is no longer constant. For every forward reaction molecules of A are destroyed. For every reverse reaction
molecules of A are destroyed. For every reverse reaction  molecules of A are created. The change in number of moles of A is then
molecules of A are created. The change in number of moles of A is then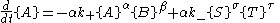
and similarly for the other reactant and products.
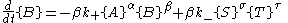
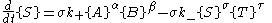
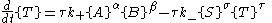
This system of equations has a single stable fixed point
Fixed point (mathematics)
In mathematics, a fixed point of a function is a point that is mapped to itself by the function. A set of fixed points is sometimes called a fixed set...
when the forward rates and the reverse rates are equal. This means that the system evolves to the equilibrium state, and this is the only state to which it evolves.
Autocatalytic reactions
Autocatalytic reactions are those in which at least one of the products is a reactant. Perhaps the simplest autocatalytic reaction can be written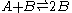
with the rate equations
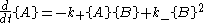
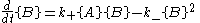 .
.This reaction is one in which a molecule of species A interacts with a molecule of species B. The A molecule is converted into a B molecule. The final product consists of the original B molecule plus the B molecule created in the reaction.
The key feature of these rate equations is that they are nonlinear; the second term on the right varies as the square of the concentration of B. This feature can lead to multiple fixed points of the system, much like a quadratic equation
Quadratic equation
In mathematics, a quadratic equation is a univariate polynomial equation of the second degree. A general quadratic equation can be written in the formax^2+bx+c=0,\,...
can have multiple roots. Multiple fixed points allow for multiple states of the system. A system existing in multiple macroscopic
Macroscopic
The macroscopic scale is the length scale on which objects or processes are of a size which is measurable and observable by the naked eye.When applied to phenomena and abstract objects, the macroscopic scale describes existence in the world as we perceive it, often in contrast to experiences or...
states is more orderly (has lower entropy) than a system in a single state.
Idealized example: Lotka-Volterra equation

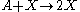
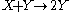
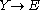
with the rate equations
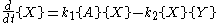
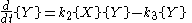 .
.Here, we have neglected the depletion of the reactant A, since its concentration is so large. The rate constants for the three reactions are
 ,
, 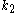 , and
, and  , respectively.
, respectively.This system of rate equations is known as the Lotka-Volterra equation
Lotka-Volterra equation
The Lotka–Volterra equations, also known as the predator–prey equations, are a pair of first-order, non-linear, differential equations frequently used to describe the dynamics of biological systems in which two species interact, one a predator and one its prey...
and is most closely associated with population dynamics
Population dynamics
Population dynamics is the branch of life sciences that studies short-term and long-term changes in the size and age composition of populations, and the biological and environmental processes influencing those changes...
in predator-prey relationships. This system of equations has an oscillatory behavior. The amplitude of the oscillations depends on the concentration of A. Oscillations of this type are a form of emergent temporal order that is not present in equilibrium.
Another idealized example: Brusselator
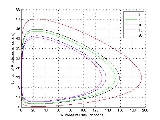
Another example of a system that demonstrates temporal order is the BrusselatorBrusselator
[Image:080205 Brusselator picture.jpg|thumb|right|350px|The Brusselator in the unstable regime. A=1. B=2.5. X=1. Y=0. The system approaches a limit cycle For B1+A^2 \,...
(see Prigogine reference). It is characterized by the reactions
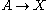
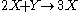
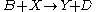
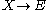
with the rate equations
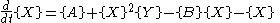
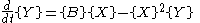
where, for convenience, the rate constants have been set to 1.
The Brusselator has a fixed point at
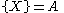
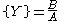 .
.The fixed point becomes unstable when
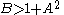
leading to an oscillation of the system. Unlike the Lotka-Volterra equation, the oscillations of the Brusselator do not depend on the amount of reactant present initially. Instead, after sufficient time, the oscillations approach a limit cycle.
Real examples
Real examples of clock reactions are the Belousov-Zhabotinsky reactionBelousov-Zhabotinsky reaction
A Belousov–Zhabotinsky reaction, or BZ reaction, is one of a class of reactions that serve as a classical example of non-equilibrium thermodynamics, resulting in the establishment of a nonlinear chemical oscillator. The only common element in these oscillating systems is the inclusion of bromine...
(BZ reaction), the Briggs-Rauscher reaction
Briggs-Rauscher reaction
The Briggs–Rauscher oscillating reaction is one of a small number of known oscillating chemical reactions. It is especially well suited for demonstration purposes because of its visually striking colour changes: the freshly prepared colourless solution slowly turns an amber colour, suddenly...
, the Bray-Liebhafsky reaction
Bray-Liebhafsky reaction
The Bray–Liebhafsky reaction is a chemical clock first described by William C. Bray in 1921 and the first oscillating reaction in a stirred homogeneous solution. He investigated the role of the iodate , the anion of iodic acid in the catalytic conversion of hydrogen peroxide to oxygen and water by...
and the iodine clock reaction
Iodine clock reaction
The iodine clock reaction is a classical chemical clock demonstration experiment to display chemical kinetics in action; it was discovered by Hans Heinrich Landolt in 1886. Two colorless solutions are mixed and at first there is no visible reaction...
. These are oscillatory reactions, and the concentration of products and reactants can be approximated in terms of damped
Damping
In physics, damping is any effect that tends to reduce the amplitude of oscillations in an oscillatory system, particularly the harmonic oscillator.In mechanics, friction is one such damping effect...
oscillation
Oscillation
Oscillation is the repetitive variation, typically in time, of some measure about a central value or between two or more different states. Familiar examples include a swinging pendulum and AC power. The term vibration is sometimes used more narrowly to mean a mechanical oscillation but sometimes...
s.
The best-known reaction, the BZ reaction, can be created with a mixture of potassium bromate
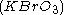 , malonic acid
, malonic acid  , and manganese sulfate
, and manganese sulfate 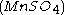 prepared in a heated solution of sulfuric acid
prepared in a heated solution of sulfuric acid 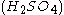 .
.Spatial order
An idealized example of spatial spontaneous symmetry breakingSpontaneous symmetry breaking
Spontaneous symmetry breaking is the process by which a system described in a theoretically symmetrical way ends up in an apparently asymmetric state....
is the case in which we have two boxes of material separated by a permeable membrane so that material can diffuse
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
between the two boxes. It is assumed that identical Brusselators are in each box with nearly identical initial conditions. (see Prigogine reference)
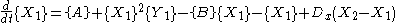
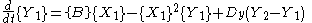
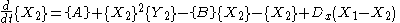
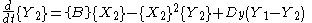
Here, the numerical subscripts indicate which box the material is in. There are additional terms proportional to the diffusion coefficient D that account for the exchange of material between boxes.
If the system is initiated with the same conditions in each box, then a small fluctuation will lead to separation of materials between the two boxes. One box will have a predominance of X, and the other will have a predominance of Y.
Biological example
It is known that an important metabolic cycle, glycolysisGlycolysis
Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+...
, displays temporal order. Glycolysis consists of the degradation of one molecule of glucose and the overall production of two molecules of ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
. The process is therefore of great importance to the energetics of living cells. The global glycolysis reaction involves glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
, ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
, NAD
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved...
, pyruvate, ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, and NADH.
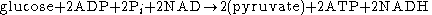 .
.The details of the process are quite involved, however, a section of the process is autocatalyzed by Phosphofructokinase (PFK). This portion of the process is responsible for oscillations in the pathway that leads to the ability of the process to oscillate between an active and an inactive form. In other words, the autocatalytic reactions can turn the process off and on.
Phase transitions
The initial amounts of reactants determine the distance from chemical equilibrium of the system. The greater the initial concentrations the further the system is from equilibrium. As the initial concentration increases, an abrupt change in orderEntropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
occurs. This abrupt change is known as phase transition
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
. At the phase transition fluctuations in macroscopic quantities, such as chemical concentrations, increase as the system oscillates between the more ordered state (lower entropy, such as ice water) and the more disordered state (higher entropy, such as liquid water). Also, at the phase transition, macroscopic equations, such as the rate equations, fail. Rate equations can be derived from microscopic considerations. The derivations typically rely on a mean field theory
Mean field theory
Mean field theory is a method to analyse physical systems with multiple bodies. A many-body system with interactions is generally very difficult to solve exactly, except for extremely simple cases . The n-body system is replaced by a 1-body problem with a chosen good external field...
approximation to microscopic dynamical equations. Mean field theory breaks down in the presence of large fluctuations (see Mean field theory
Mean field theory
Mean field theory is a method to analyse physical systems with multiple bodies. A many-body system with interactions is generally very difficult to solve exactly, except for extremely simple cases . The n-body system is replaced by a 1-body problem with a chosen good external field...
article for a discussion). Therefore, since large fluctuations occur in the neighborhood of a phase transition, macroscopic equations, such as rate equations, fail. As the initial concentration increases further, the system settles into an ordered state in which fluctuations are again small. (see Prigogine reference)
See also
- AutocatalysisAutocatalysisA single chemical reaction is said to have undergone autocatalysis, or be autocatalytic, if the reaction product itself is the catalyst for that reaction....
- Catalytic cycleCatalytic cycleA catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
- Reaction-diffusion
- AbiogenesisAbiogenesisAbiogenesis or biopoesis is the study of how biological life arises from inorganic matter through natural processes, and the method by which life on Earth arose...
- Stuart KauffmanStuart KauffmanStuart Alan Kauffman is an American theoretical biologist and complex systems researcher concerning the origin of life on Earth...
- MorphogenesisMorphogenesisMorphogenesis , is the biological process that causes an organism to develop its shape...

