Autocatalysis
Overview
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
is said to have undergone autocatalysis, or be autocatalytic, if the reaction product itself is the catalyst for that reaction.
A set of chemical reactions can be said to be "collectively autocatalytic" if a number of those reactions produce, as reaction products, catalysts for enough of the other reactions that the entire set of chemical reactions is self sustaining given an input of energy and food molecules (see autocatalytic set
Autocatalytic set
An autocatalytic set is a collection of entities, each of which can be created catalytically by other entities within the set, such that as a whole, the set is able to catalyze its own production. In this way the set as a whole is said to be autocatalytic...
).
The rate law for the second order autocatalytic reaction
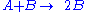
is the following one
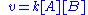 .
.The concentrations of A and B vary in time according to

and
 .
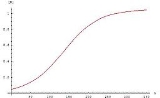
.The graph for these equations is a sigmoid curve
Sigmoid function
Many natural processes, including those of complex system learning curves, exhibit a progression from small beginnings that accelerates and approaches a climax over time. When a detailed description is lacking, a sigmoid function is often used. A sigmoid curve is produced by a mathematical...
, which is typical for autocatalytic reactions: these chemical reactions proceed slowly at the start because there is little catalyst present, the rate of reaction increases progressively as the reaction proceeds as the amount of catalyst increases and then it again slows down as the reactant concentration decreases.
Unanswered Questions

