Atropisomer
Encyclopedia

Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
s where the steric strain barrier to rotation is high enough to allow for the isolation of the conformers. The word atropisomer is derived from the Greek
Greek language
Greek is an independent branch of the Indo-European family of languages. Native to the southern Balkans, it has the longest documented history of any Indo-European language, spanning 34 centuries of written records. Its writing system has been the Greek alphabet for the majority of its history;...
a, meaning not, and tropos, meaning turn. The name was coined by Kuhn in 1933, but atropisomerism was first detected in 6,6’-dinitro-2,2’-diphenic acid by Cristie in 1922.
Oki defined atropisomers as conformers that interconvert with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of more than 1000 seconds at a given temperature. Atropisomers are an important class of compounds because they display axial chirality
Axial chirality
Axial chirality is a special case of chirality in which a molecule does not possess a stereogenic center but an axis of chirality – an axis about which a set of substituents is held in a spatial arrangement that is not superposable on its mirror image...
. They differ from other chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
compounds in that they can be equilibrated
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
thermally whereas in the other forms of chirality isomerization is usually only possible chemically.
The most important class of atropisomers are biaryls
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
such as diphenic acid, which is a derivative of biphenyl
Biphenyl
Biphenyl is an organic compound that forms colorless crystals. It has a distinctively pleasant smell. Biphenyl is an aromatic hydrocarbon with a molecular formula 2...
with a complete set of ortho
Arene substitution patterns
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.- Ortho, meta, and para substitution :...
substituents. Others are dimers of naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
derivatives such as 1,1'-bi-2-naphthol
1,1'-Bi-2-naphthol
1,1'-Bi-2-naphthol is an organic compound that is often used as a ligand for transition-metal catalysed asymmetric synthesis. BINOL has axial chirality and the two enantiomers can be readily separated and are stable toward racemisation. The specific rotation of the two enantiomers is +/- 35.5°...
. In a similar way, aliphatic ring systems like cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
s linked through a single bond may display atropisomerism provided that bulky substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s are present.
Examples of naturally occurring atropisomers include vancomycin
Vancomycin
Vancomycin INN is a glycopeptide antibiotic used in the prophylaxis and treatment of infections caused by Gram-positive bacteria. It has traditionally been reserved as a drug of "last resort", used only after treatment with other antibiotics had failed, although the emergence of...
and knipholone, which is found in the roots of Kniphofia Foliosa of the family Asphodelaceae
Asphodelaceae
Asphodeloideae is a subfamily of the monocot family Xanthorrhoeaceae in the order Asparagales. It has previously been treated as a separate family, Asphodelaceae. The subfamily name is derived from the generic name of the type genus, Asphodelus...
.
Separation of atropisomers is possibly by chiral resolution
Chiral resolution
Chiral resolution in stereochemistry is a process for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active drugs...
methods such as selective crystallization. In an atropo-enantioselective or atropselective synthesis one atropisomer is formed at the expense of the other. Atroposelective synthesis may be carried out by use of chiral auxiliaries
Chiral auxiliary
A chiral auxiliary is a chemical compound or unit that is temporarily incorporated into an organic synthesis so that it can be carried out asymmetrically with the selective formation of one of two enantiomers...
like a CBS catalyst
CBS catalyst
The CBS catalyst or Corey-Bakshi-Shibata catalyst is an asymmetric catalyst derived from proline. It finds many uses in organic reactions such as the CBS reduction, Diels-Alder reactions and [3+2] cycloadditions. Proline, a naturally occurring chiral compound, is readily and cheaply available...
in the total synthesis
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of knipholone or by approaches based on thermodynamic equilibration when an isomerization reaction favors one atropisomer over the other.
In organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
BINAP
BINAP
BINAP is an abbreviation for the organophosphorus compound 2,2'-bis-1,1'-binaphthyl. This chiral ligand is widely used in asymmetric synthesis. It consists of a pair of 2-diphenylphosphinonaphthyl groups linked at the 1 and 1´ positions. This C2-symmetric framework lacks stereogenic atom, but...
is a ligand that is used in the preparation of optically active stereoisomers.
Scope
In one application the asymmetry in an atropisomer is transferred in a chemical reaction to a new stereocenterStereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
. The atropisomer is an iodoaryl compound synthesised starting from (S)-valine
Valine
Valine is an α-amino acid with the chemical formula HO2CCHCH2. L-Valine is one of 20 proteinogenic amino acids. Its codons are GUU, GUC, GUA, and GUG. This essential amino acid is classified as nonpolar...
and exists as the (M,S) isomer and the (P,S) isomer. The interconversion barrier between the two is 24.3 kcal/mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
(101.7 kJ/mol). The (M,S) isomer can be obtained exclusively from this mixture by recrystallisation
Crystallization
Crystallization is the process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid...
from hexane
Hexane
Hexane is a hydrocarbon with the chemical formula C6H14; that is, an alkane with six carbon atoms.The term may refer to any of four other structural isomers with that formula, or to a mixture of them. In the IUPAC nomenclature, however, hexane is the unbranched isomer ; the other four structures...
s. The iodine group is homolytically
Homolysis
In general it means breakdown to equal pieces There are separate meanings for the word in chemistry and biology.-Homolysis in chemistry:...
removed to form an aryl radical
Aryl radical
An Aryl radical in organic chemistry is an reactive intermediate and an arene compound incorporating one free radical carbon atom as part of the ring structure. As such it is the radical counterpart of the Arenium ion. The parent compound is the phenyl radical C6H5....
by a tributyltin hydride
Organotin
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849...
/triethylboron/oxygen mixture as in the Barton-McCombie reaction. Although the hindered rotation is now removed in the aryl radical the intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
reaction with the alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
is so much faster than rotation of the carbon-nitrogen bond
Carbon-nitrogen bond
A carbon–nitrogen bond is a covalent bond between carbon and nitrogen and is one of the most abundant bonds in organic chemistry and biochemistry....
that the stereochemistry is preserved. In this way the (M,S) isomer yields the (S,S) dihydroindolone.
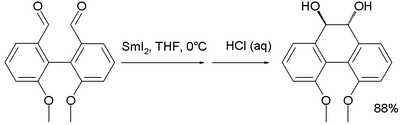
An axial chirality switch is reported for a diol prepared from intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
pinacol coupling of the corresponding di-aldehyde with samarium(II) iodide
Samarium(II) iodide
Samarium iodide is a green solid composed of samarium and iodine, with a melting point of 520 °C where the samarium atom has a coordination number of seven in a capped octahedral configuration...
. In methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
this compound has the two alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
groups in equatorial positions but in hexane
Hexane
Hexane is a hydrocarbon with the chemical formula C6H14; that is, an alkane with six carbon atoms.The term may refer to any of four other structural isomers with that formula, or to a mixture of them. In the IUPAC nomenclature, however, hexane is the unbranched isomer ; the other four structures...
helicity
Helicity
The term helicity has several meanings. In physics, all referring to a phenomenon that resembles a helix. See:*helicity , the extent to which corkscrew-like motion occurs...
is reversed with both groups in axial positions.
Drugs
TelenzepineTelenzepine
Telenzepine is an anticholinergic or sympatholytic used in the treatment of peptic ulcers. Telenzepine is atropisomeric, in other words the molecule has a stereogenic C–N-axis in neutral aqueous solution it displays a half-life for racemization of the order of 1000 years. The enantiomers have been...
is atropisomeric, in other words the molecule has a stereogenic C–N-axis in neutral aqueous solution it displays a half-life for racemization of the order of 1000 years. The enantiomers have been resolved. The activity is related to the (+)-isomer which has about 500-fold more active as the (–)-isomer at muscarinic receptors in rat cerebal cortex.