
Atomic form factor
Encyclopedia
In physics
, the atomic form factor, or atomic scattering factor, is a measure of the scattering amplitude
of a wave by an isolated atom. The atomic form factor depends on the type of scattering, which in turn depends on the nature of the incident radiation, typically X-ray, electron
or neutron
. The common feature of all form factors is that they involve a Fourier transform
of a spatial density distribution of the scattering object from real space
to k-space
(also known as reciprocal space). For an object that is spherically symmetric, the spatial density distribution can be expressed as a function of radius,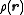 , so that the form factor,
, so that the form factor, 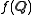 is defined as :
is defined as :
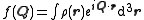 ,
,
where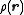 is the spatial density of the scatterer about the center of mass
is the spatial density of the scatterer about the center of mass
of the scatterer (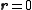 ), and
), and 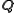 is the momentum transfer. As a result of the nature of the Fourier transform, the broader the distribution of the scatterer
is the momentum transfer. As a result of the nature of the Fourier transform, the broader the distribution of the scatterer 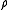 in real space
in real space  , the narrower the distribution of
, the narrower the distribution of 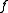 in
in 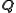 ; i.e., the faster the decay of the form factor.
; i.e., the faster the decay of the form factor.
For crystals, atomic form factors are used to calculate the structure factor
for a given Bragg peak
of a crystal
.
,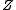 , of the atoms in a sample. As a result X-rays are not very sensitive to light atoms, such as hydrogen
, of the atoms in a sample. As a result X-rays are not very sensitive to light atoms, such as hydrogen
and helium
, and there is very little contrast between elements adjacent to each other in the periodic table
. For X-ray scattering,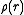 in the above equation is the electron
in the above equation is the electron
charge density
about the nucleus, and the form factor the Fourier transform of this quantity. The assumption of a spherical distribution is usually good enough for X-ray crystallography
.
In general the X-ray form factor is complex but the imaginary components only become large near an absorption edge
. Anomalous X-ray scattering
makes use of the variation of the form factor close to an absorption edge to vary the scattering power of specific atoms in the sample by changing the energy of the incident x-rays hence enabling the extraction of more detailed structural information.
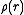 is the potential distribution
is the potential distribution
of the atom, and the electron form factor is the Fourier transform of this. The electron form factors are normally calculated from X-ray form factors using the Mott-Bethe formula. This formula takes into account both elastic electron-cloud scattering and elastic nuclear scattering.
. Both are used in the investigation structure and dynamics of condensed matter
: they are termed nuclear (sometimes also termed chemical) and magnetic scattering.
of thermal (several Angstroms) and cold neutrons (up to tens of Angstroms) typically used for such investigations is 4-5 orders of magnitude larger than the dimension of the nucleus (femtometres). The free neutrons in a beam
travel in a plane wave
; for those that undergo nuclear scattering from a nucleus, the nucleus acts as a secondary point source
, and radiates scattered neutrons as a spherical wave. (Although a quantum phenomenon, this can be visualized in simple classical terms by the Huygens–Fresnel principle.) In this case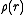 is the spatial density distribution of the nucleus, which is an infinitesimal point (delta function
is the spatial density distribution of the nucleus, which is an infinitesimal point (delta function
), with respect to the neutron wavelength. The delta function forms part of the Fermi pseudopotential, by which the free neutron and the nuclei interact. The Fourier transform of a delta function is unity; therefore, it is commonly said that neutrons "do not have a form factor;" i.e., the scattered amplitude,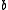 , is independent of
, is independent of 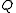 .
.
Since the interaction is nuclear, each isotope has a different scattering amplitude. This Fourier transform is scaled by the amplitude
of the spherical wave, which has dimensions of length. Hence, the amplitude of scattering that characterizes the interaction of a neutron with a given isotope is termed the scattering length, b. Neutron scattering lengths vary erratically between neighbouring elements in the periodic table
and between isotopes of the same element. They may only be determined experimentally, since the theory of nuclear forces is not adequate to calculate or predict b from other properties of the nucleus.
and hence have an associated magnetic moment
. In neutron scattering from condensed matter, magnetic scattering refers to the interaction of this moment with the magnetic moments arising from unpaired electrons in the outer orbitals
of certain atoms. It is the spatial distribution of these unpaired electrons about the nucleus that is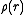 for magnetic scattering.
for magnetic scattering.
Since these orbitals are typically of a comparable size to the wavelength of the free neutrons, the resulting form factor resembles that of the X-ray form factor. However, this neutron-magnetic scattering is only from the outer electrons, rather than being heavily weighted by the core electrons, which is the case for X-ray scattering. Hence, in strong contrast to the case for nuclear scattering, the scattering object for magnetic scattering is far from a point source; it is still more diffuse than the effective size of the source for X-ray scattering, and the resulting Fourier transform (the magnetic form factor) decays more rapidly than the X-ray form factor. Also, in contrast to nuclear scattering, the magnetic form factor is not isotope dependent, but is dependent on the oxidation state of the atom.
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
, the atomic form factor, or atomic scattering factor, is a measure of the scattering amplitude
Scattering amplitude
In quantum physics, the scattering amplitude is the amplitude of the outgoing spherical wave relative to the incoming plane wave in the stationary-state scattering process...
of a wave by an isolated atom. The atomic form factor depends on the type of scattering, which in turn depends on the nature of the incident radiation, typically X-ray, electron
Electron diffraction
Electron diffraction refers to the wave nature of electrons. However, from a technical or practical point of view, it may be regarded as a technique used to study matter by firing electrons at a sample and observing the resulting interference pattern...
or neutron
Neutron diffraction
Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material: A sample to be examined is placed in a beam of thermal or cold neutrons to obtain a diffraction pattern that provides information of...
. The common feature of all form factors is that they involve a Fourier transform
Fourier transform
In mathematics, Fourier analysis is a subject area which grew from the study of Fourier series. The subject began with the study of the way general functions may be represented by sums of simpler trigonometric functions...
of a spatial density distribution of the scattering object from real space
Real space
Position space denominates the space of possible locations of an object in classical physics.The real space coordinates specify the position of an object. For instance,...
to k-space
K-space
K-space can refer to:*Another name for the Frequency domain but referring to a spatial rather than temporal frequency*Reciprocal space for the Fourier transform of a spatial function...
(also known as reciprocal space). For an object that is spherically symmetric, the spatial density distribution can be expressed as a function of radius,
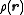 , so that the form factor,
, so that the form factor, 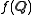 is defined as :
is defined as :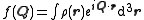 ,
,where
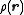 is the spatial density of the scatterer about the center of mass
is the spatial density of the scatterer about the center of massCenter of mass
In physics, the center of mass or barycenter of a system is the average location of all of its mass. In the case of a rigid body, the position of the center of mass is fixed in relation to the body...
of the scatterer (
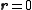 ), and
), and 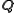 is the momentum transfer. As a result of the nature of the Fourier transform, the broader the distribution of the scatterer
is the momentum transfer. As a result of the nature of the Fourier transform, the broader the distribution of the scatterer 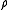 in real space
in real space  , the narrower the distribution of
, the narrower the distribution of 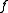 in
in 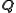 ; i.e., the faster the decay of the form factor.
; i.e., the faster the decay of the form factor.For crystals, atomic form factors are used to calculate the structure factor
Structure factor
In condensed matter physics and crystallography, the static structure factor is a mathematical description of how a material scatters incident radiation...
for a given Bragg peak
Bragg Peak
The Bragg peak is a pronounced peak on the Bragg curve which plots the energy loss of ionizing radiation during its travel through matter. For protons, α-rays, and other ion rays, the peak occurs immediately before the particles come to rest...
of a crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
.
X-ray form factor
X-rays are scattered by the electron cloud of the atom and hence the scattering amplitude of X-rays increases with the atomic numberAtomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
,
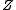 , of the atoms in a sample. As a result X-rays are not very sensitive to light atoms, such as hydrogen
, of the atoms in a sample. As a result X-rays are not very sensitive to light atoms, such as hydrogenHydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
, and there is very little contrast between elements adjacent to each other in the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
. For X-ray scattering,
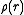 in the above equation is the electron
in the above equation is the electronElectron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
charge density
Charge density
The linear, surface, or volume charge density is the amount of electric charge in a line, surface, or volume, respectively. It is measured in coulombs per meter , square meter , or cubic meter , respectively, and represented by the lowercase Greek letter Rho . Since there are positive as well as...
about the nucleus, and the form factor the Fourier transform of this quantity. The assumption of a spherical distribution is usually good enough for X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
.
In general the X-ray form factor is complex but the imaginary components only become large near an absorption edge
Absorption edge
An absorption edge is a sharp discontinuity in the absorption spectrum of a material. These discontinuities occur at wavelengths at which the energy of an absorbed photon corresponds to an electronic transition or ionisation potential....
. Anomalous X-ray scattering
Anomalous X-ray scattering
Anomalous X-ray scattering AXRS or XRAS is a technique within X-ray diffraction that makes use of the anomalous dispersion that occurs when a wavelength is selected that is in the vicinity of an absorption edge of one of the constituent elements of the sample.-Atomic scattering factors:In X-ray...
makes use of the variation of the form factor close to an absorption edge to vary the scattering power of specific atoms in the sample by changing the energy of the incident x-rays hence enabling the extraction of more detailed structural information.
Electron form factor
The relevant distribution,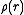 is the potential distribution
is the potential distributionElectric potential
In classical electromagnetism, the electric potential at a point within a defined space is equal to the electric potential energy at that location divided by the charge there...
of the atom, and the electron form factor is the Fourier transform of this. The electron form factors are normally calculated from X-ray form factors using the Mott-Bethe formula. This formula takes into account both elastic electron-cloud scattering and elastic nuclear scattering.
Neutron form factor
There are two distinct scattering interactions of neutrons by nucleiAtomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
. Both are used in the investigation structure and dynamics of condensed matter
Condensed Matter
Condensed matter may refer to several things*Condensed matter physics, the study of the physical properties of condensed phases of matter*European Physical Journal B: Condensed Matter and Complex Systems, a scientific journal published by EDP sciences...
: they are termed nuclear (sometimes also termed chemical) and magnetic scattering.
Nuclear scattering
Nuclear scattering of the free neutron by the nucleus is mediated by the strong nuclear force. The wavelengthWavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
of thermal (several Angstroms) and cold neutrons (up to tens of Angstroms) typically used for such investigations is 4-5 orders of magnitude larger than the dimension of the nucleus (femtometres). The free neutrons in a beam
Particle beam
A particle beam is a stream of charged or neutral particles which may be directed by magnets and focused by electrostatic lenses, although they may also be self-focusing ....
travel in a plane wave
Plane wave
In the physics of wave propagation, a plane wave is a constant-frequency wave whose wavefronts are infinite parallel planes of constant peak-to-peak amplitude normal to the phase velocity vector....
; for those that undergo nuclear scattering from a nucleus, the nucleus acts as a secondary point source
Point source
A point source is a localised, relatively small source of something.Point source may also refer to:*Point source , a localised source of pollution**Point source water pollution, water pollution with a localized source...
, and radiates scattered neutrons as a spherical wave. (Although a quantum phenomenon, this can be visualized in simple classical terms by the Huygens–Fresnel principle.) In this case
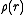 is the spatial density distribution of the nucleus, which is an infinitesimal point (delta function
is the spatial density distribution of the nucleus, which is an infinitesimal point (delta functionDirac delta function
The Dirac delta function, or δ function, is a generalized function depending on a real parameter such that it is zero for all values of the parameter except when the parameter is zero, and its integral over the parameter from −∞ to ∞ is equal to one. It was introduced by theoretical...
), with respect to the neutron wavelength. The delta function forms part of the Fermi pseudopotential, by which the free neutron and the nuclei interact. The Fourier transform of a delta function is unity; therefore, it is commonly said that neutrons "do not have a form factor;" i.e., the scattered amplitude,
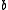 , is independent of
, is independent of 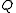 .
.Since the interaction is nuclear, each isotope has a different scattering amplitude. This Fourier transform is scaled by the amplitude
Scattering amplitude
In quantum physics, the scattering amplitude is the amplitude of the outgoing spherical wave relative to the incoming plane wave in the stationary-state scattering process...
of the spherical wave, which has dimensions of length. Hence, the amplitude of scattering that characterizes the interaction of a neutron with a given isotope is termed the scattering length, b. Neutron scattering lengths vary erratically between neighbouring elements in the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
and between isotopes of the same element. They may only be determined experimentally, since the theory of nuclear forces is not adequate to calculate or predict b from other properties of the nucleus.
Magnetic scattering
Although neutral, neutrons also have a nuclear spin. They are a composite fermionFermion
In particle physics, a fermion is any particle which obeys the Fermi–Dirac statistics . Fermions contrast with bosons which obey Bose–Einstein statistics....
and hence have an associated magnetic moment
Magnetic moment
The magnetic moment of a magnet is a quantity that determines the force that the magnet can exert on electric currents and the torque that a magnetic field will exert on it...
. In neutron scattering from condensed matter, magnetic scattering refers to the interaction of this moment with the magnetic moments arising from unpaired electrons in the outer orbitals
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
of certain atoms. It is the spatial distribution of these unpaired electrons about the nucleus that is
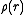 for magnetic scattering.
for magnetic scattering.Since these orbitals are typically of a comparable size to the wavelength of the free neutrons, the resulting form factor resembles that of the X-ray form factor. However, this neutron-magnetic scattering is only from the outer electrons, rather than being heavily weighted by the core electrons, which is the case for X-ray scattering. Hence, in strong contrast to the case for nuclear scattering, the scattering object for magnetic scattering is far from a point source; it is still more diffuse than the effective size of the source for X-ray scattering, and the resulting Fourier transform (the magnetic form factor) decays more rapidly than the X-ray form factor. Also, in contrast to nuclear scattering, the magnetic form factor is not isotope dependent, but is dependent on the oxidation state of the atom.

