Aspartate carbamoyltransferase
Encyclopedia
Aspartate carbamoyltransferase (also known as aspartate transcarbamoylase or ATCase) catalyzes the first step in the pyrimidine biosynthetic pathway .
In E. coli, the enzyme is a multi-subunit
protein
complex composed of 12 subunits (300 kDa in total). The composition of the subunits is C6R6, forming 2 trimer
s of catalytic subunits (34 kDa) and 3 dimer
s of regulatory subunits (17 kDa). The particular arrangement of catalytic and regulatory subunits in this enzyme affords the complex with strongly allosteric behaviour with respect to its substrates. The enzyme is an archetypal example of allosteric modulation of fine control of metabolic enzyme reactions.
ATCase does not follow Michaelis-Menten kinetics, but lies between the low-activity, low-affinity "tight" or T and the high-activity, high-affinity "relaxed" or R states. The binding of substrate to the catalytic subunits results in an equilibrium shift towards the R state, whereas binding of CTP to the regulatory subunits results in an equilibrium shift towards the T state. Binding of ATP to the regulatory subunits results in an equilibrium shift towards the R state.
s and purine
s. The end-product of the pyrimidine pathway, CTP
, induces a decrease in catalytic velocity, whereas ATP
, the end-product of the parallel purine pathway, exerts the opposite effect, stimulating the catalytic activity.
.jpg) (The discussion of structure, catalytic center, and allosteric site that follows is based on the prokaryotic version of ATCase, to be specific, from E. coli.)
(The discussion of structure, catalytic center, and allosteric site that follows is based on the prokaryotic version of ATCase, to be specific, from E. coli.)
Early studies demonstrated that ATCase consists of two different kinds of polypeptide chains, which have different roles. The catalytic subunits catalyze the carbamylation of the amino group of aspartate but do not have regulatory properties, while the regulatory subunits do not have any catalytic activity but contain the regulatory site
s for effector binding. The ATCase holoenzyme is made of two catalytic trimers that are in contact and held together by three regulatory dimers, so the native form of the enzyme contains six chains of each type, with a total molecular weight
of 310 kDa
.
Each of the catalytic domains is composed of two structural domains, the aspartate domain, which contains most of the residues responsible for binding aspartate, and the carbamoyl phosphate domain, which contains most of the residues that bind to carbamoyl phosphate
. Each regulatory domain is also composed of two domains, the allosteric domain, which has the binding site for the nucleotide effectors
, and the zinc
domain, consisting of four cysteine
residues clustered in its C-terminal region. These residues coordinate a zinc
atom
that is not involved in any catalytic property, but has been shown to be essential for the association of regulatory and catalytic subunits.
The three-dimensional arrangement of the catalytic and regulatory subunits involves several ionic and hydrophobic stabilizing contacts between amino acid
residues. Each catalytic chain is in contact with three other catalytic chains and two regulatory chains. Each regulatory monomer is in contact with one other regulatory chain and two catalytic chains. In the unliganded enzyme, the two catalytic trimers are also in contact.
of the substrates. Additionally, crystal structures of ATCase bound to carbamoylphosphate and succinate have been obtained. These studies, in addition to investigations using site-directed mutagenesis of specific amino acids, have identified several residues that are crucial for catalysis, such as Ser52, Thr53, Arg54, Thr55, Arg105, His134, Gln137, Arg167, Arg229, Glu231, and Ser80 and Lys84 from an adjacent catalytic chain. The active site is a highly positively charged pocket. One of the most critical side-chains is from Arg54, which interacts with a terminal oxygen and the anhydride oxygen of carbamoyl phosphate, stabilizing the negative charge of the leaving phosphate group. Arg105, His134, and Thr55 help to increase the electrophilicity of the carbonyl carbon by interacting with the carbonyl oxygen. In general, the rate enhancement of ATCase is achieved by orientation and stabilization of substrates, intermediates, and products rather than by direct involvement of amino acid residues in the catalytic mechanism.
Comparison of the crystal structures of the T and R forms of ATCase show that it swells in size during the allosteric transition, and that the catalytic subunits condense during this process. The two catalytic trimers move apart along the threefold axis by 12 Å, and they rotate about this axis by 5° each, ultimately leading to a reorientation of the regulatory subunits around their twofold axis by 15°. This quaternary structure
change is associated with alterations in inter-subunit and inter-domain interactions. The interaction between subunits C1-C4 and R1 is extensively modified during this conversion. In particular, there is large movement of amino acid residues 230-254, known collectively as the 240s loop. These residues are located at the cleft between the carbamoyl phosphate
and aspartate domains at the C1-C4 interface. The overall outcome of these structural changes is that the two domains of each catalytic chain come closer together, ensuring a better contact with the substrates
or their analogues
.
During this structural transition, some interactions between side-chains are lost and some others are established. Studies have confirmed that the position of the 240s loop directly affects substrate binding in the corresponding active site. Earlier studies using site-directed mutagenesis of the 240s loop showed that interactions between Asp271 and Tyr240, and between Glu239 of C1 and Tyr165 of C4 would stabilize the T-state, while interactions between Glu239 of C1 and both Lys164 and Tyr165 of C4 would stabilize the R-state.
Located close to the 240s loop and the active site, the loop region encompassing residues 160-166 plays a role in both the internal architecture of the enzyme and its regulatory properties. In particular, the residue Asp162 interacts with Gln231 (known to be involved in aspartate binding), and binds the same residues in both the T and R states. A mutant that had this residue mutated to alanine
showed a huge reduction in specific activity, a two-fold decrease in the affinity for aspartate, a loss of homotropic cooperativity, and decreased activation by ATP
. It was suggested that the change in the overall structure caused by the introduction of this residue affects other residues in the R1-C1, R1-C4 and C1-C4 interfaces, which are involved in the quaternary structure
transition.
In E. coli, the enzyme is a multi-subunit
Protein subunit
In structural biology, a protein subunit or subunit protein is a single protein molecule that assembles with other protein molecules to form a protein complex: a multimeric or oligomeric protein. Many naturally occurring proteins and enzymes are multimeric...
protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
complex composed of 12 subunits (300 kDa in total). The composition of the subunits is C6R6, forming 2 trimer
Trimer (chemistry)
In chemistry, a trimer is a product derived from three identical precursors. Trimers are typically cyclic. Chemical compounds that often trimerise are aliphatic isocyanates and cyanic acids. Often, trimerization competes with polymerization....
s of catalytic subunits (34 kDa) and 3 dimer
Protein dimer
In biochemistry, a dimer is a macromolecular complex formed by two, usually non-covalently bound, macromolecules like proteins or nucleic acids...
s of regulatory subunits (17 kDa). The particular arrangement of catalytic and regulatory subunits in this enzyme affords the complex with strongly allosteric behaviour with respect to its substrates. The enzyme is an archetypal example of allosteric modulation of fine control of metabolic enzyme reactions.
ATCase does not follow Michaelis-Menten kinetics, but lies between the low-activity, low-affinity "tight" or T and the high-activity, high-affinity "relaxed" or R states. The binding of substrate to the catalytic subunits results in an equilibrium shift towards the R state, whereas binding of CTP to the regulatory subunits results in an equilibrium shift towards the T state. Binding of ATP to the regulatory subunits results in an equilibrium shift towards the R state.
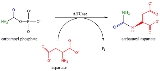
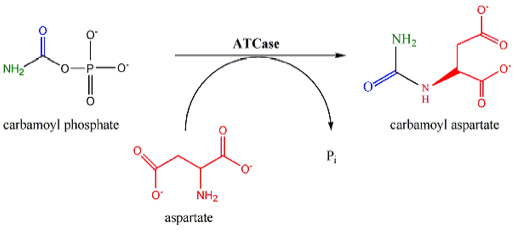
Reaction
ATCase is a highly regulated enzyme that catalyses the first committed step in pyrimidine biosynthesis, the condensation of aspartate and carbamyl phosphate to form N-carbamyl-L-aspartate and inorganic phosphate. ATCase controls the rate of pyrimidine biosynthesis by altering its catalytic velocity in response to cellular levels of both pyrimidinePyrimidine
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
s and purine
Purine
A purine is a heterocyclic aromatic organic compound, consisting of a pyrimidine ring fused to an imidazole ring. Purines, including substituted purines and their tautomers, are the most widely distributed kind of nitrogen-containing heterocycle in nature....
s. The end-product of the pyrimidine pathway, CTP
Cytidine triphosphate
Cytidine triphosphate is a pyrimidine nucleoside triphosphate.CTP is a substrate in the synthesis of RNA.CTP is a high-energy molecule equal to ATP, but its role in the organism is more specific than that of ATP....
, induces a decrease in catalytic velocity, whereas ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, the end-product of the parallel purine pathway, exerts the opposite effect, stimulating the catalytic activity.

Structure
.jpg)
Early studies demonstrated that ATCase consists of two different kinds of polypeptide chains, which have different roles. The catalytic subunits catalyze the carbamylation of the amino group of aspartate but do not have regulatory properties, while the regulatory subunits do not have any catalytic activity but contain the regulatory site
Regulatory site
A regulatory site is a site on an allosteric protein to which a modulator molecule binds. A ligand-binding site on a receptor or enzyme distinct from the active site. Allosteric modulators alter enzyme activity by binding to the regulatory site. Also known as an "allosteric site"....
s for effector binding. The ATCase holoenzyme is made of two catalytic trimers that are in contact and held together by three regulatory dimers, so the native form of the enzyme contains six chains of each type, with a total molecular weight
Molecular mass
The molecular mass of a substance is the mass of one molecule of that substance, in unified atomic mass unit u...
of 310 kDa
KDA
KDA may refer to:* Karachi Development Authority* Kongsberg Defence & Aerospace* Kotelawala Defence Academy* Kramer Design Associates* Lithium diisopropylamide, KDA is the potassium analogue of lithium diisopropylamideOr kDa may refer to:...
.
Each of the catalytic domains is composed of two structural domains, the aspartate domain, which contains most of the residues responsible for binding aspartate, and the carbamoyl phosphate domain, which contains most of the residues that bind to carbamoyl phosphate
Carbamoyl phosphate
Carbamoyl phosphate is an anion of biochemical significance. In land-dwelling animals it is an intermediary metabolite participating in the nitrogen disposal through in the urea cycle and the synthesis of pyrimidines....
. Each regulatory domain is also composed of two domains, the allosteric domain, which has the binding site for the nucleotide effectors
Effector (biology)
An effector is a molecule that binds to a protein and thereby alters the activity of that protein...
, and the zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
domain, consisting of four cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
residues clustered in its C-terminal region. These residues coordinate a zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
that is not involved in any catalytic property, but has been shown to be essential for the association of regulatory and catalytic subunits.
The three-dimensional arrangement of the catalytic and regulatory subunits involves several ionic and hydrophobic stabilizing contacts between amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
residues. Each catalytic chain is in contact with three other catalytic chains and two regulatory chains. Each regulatory monomer is in contact with one other regulatory chain and two catalytic chains. In the unliganded enzyme, the two catalytic trimers are also in contact.
Catalytic center
The catalytic site of ATCase is located at the interface between two neighboring catalytic chains in the same trimer and incorporates amino acid side-chains from both of these subunits. Insight into the mode of binding of substrates to the catalytic center of ATCase was first made possible by the binding of a bisubstrate analogue, N-(phosphonoacetyl)-L-aspartate (PALA). This compound is a strong inhibitor of ATCase and has a structure that is thought to be very close to that of the transition stateTransition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
of the substrates. Additionally, crystal structures of ATCase bound to carbamoylphosphate and succinate have been obtained. These studies, in addition to investigations using site-directed mutagenesis of specific amino acids, have identified several residues that are crucial for catalysis, such as Ser52, Thr53, Arg54, Thr55, Arg105, His134, Gln137, Arg167, Arg229, Glu231, and Ser80 and Lys84 from an adjacent catalytic chain. The active site is a highly positively charged pocket. One of the most critical side-chains is from Arg54, which interacts with a terminal oxygen and the anhydride oxygen of carbamoyl phosphate, stabilizing the negative charge of the leaving phosphate group. Arg105, His134, and Thr55 help to increase the electrophilicity of the carbonyl carbon by interacting with the carbonyl oxygen. In general, the rate enhancement of ATCase is achieved by orientation and stabilization of substrates, intermediates, and products rather than by direct involvement of amino acid residues in the catalytic mechanism.
Allosteric site
The allosteric site in the allosteric domain of the R chains of the ATCase complex binds to the nucleotides ATP, CTP and/or UTP. There is one site with high affinity for ATP and CTP and one with 10- to 20-fold lower affinity for these nucleotides in each regulatory dimer. ATP binds predominantly to the high-affinity sites and subsequently activates the enzyme, while UTP and CTP binding leads to inhibition of activity. UTP can bind to the allosteric site, but inhibition of ATCase by UTP is possible only in combination with CTP. With CTP present, UTP binding is enhanced and preferentially directed to the low-affinity sites. on the converse, UTP binding leads to enhanced affinity for CTP at the high-affinity sites and inhibits enzyme activity by up to 95%, while CTP binding alone inhibits activity to 50% to 70%.Comparison of the crystal structures of the T and R forms of ATCase show that it swells in size during the allosteric transition, and that the catalytic subunits condense during this process. The two catalytic trimers move apart along the threefold axis by 12 Å, and they rotate about this axis by 5° each, ultimately leading to a reorientation of the regulatory subunits around their twofold axis by 15°. This quaternary structure
Quaternary structure
In biochemistry, quaternary structure is the arrangement of multiple folded protein or coiling protein molecules in a multi-subunit complex.-Description and examples:...
change is associated with alterations in inter-subunit and inter-domain interactions. The interaction between subunits C1-C4 and R1 is extensively modified during this conversion. In particular, there is large movement of amino acid residues 230-254, known collectively as the 240s loop. These residues are located at the cleft between the carbamoyl phosphate
Carbamoyl phosphate
Carbamoyl phosphate is an anion of biochemical significance. In land-dwelling animals it is an intermediary metabolite participating in the nitrogen disposal through in the urea cycle and the synthesis of pyrimidines....
and aspartate domains at the C1-C4 interface. The overall outcome of these structural changes is that the two domains of each catalytic chain come closer together, ensuring a better contact with the substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
or their analogues
Analog (chemistry)
In chemistry, a structural analog , also known as chemical analog or simply analog, is a compound having a structure similar to that of another one, but differing from it in respect of a certain component. It can differ in one or more atoms, functional groups, or substructures, which are replaced...
.
During this structural transition, some interactions between side-chains are lost and some others are established. Studies have confirmed that the position of the 240s loop directly affects substrate binding in the corresponding active site. Earlier studies using site-directed mutagenesis of the 240s loop showed that interactions between Asp271 and Tyr240, and between Glu239 of C1 and Tyr165 of C4 would stabilize the T-state, while interactions between Glu239 of C1 and both Lys164 and Tyr165 of C4 would stabilize the R-state.
Located close to the 240s loop and the active site, the loop region encompassing residues 160-166 plays a role in both the internal architecture of the enzyme and its regulatory properties. In particular, the residue Asp162 interacts with Gln231 (known to be involved in aspartate binding), and binds the same residues in both the T and R states. A mutant that had this residue mutated to alanine
Alanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
showed a huge reduction in specific activity, a two-fold decrease in the affinity for aspartate, a loss of homotropic cooperativity, and decreased activation by ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
. It was suggested that the change in the overall structure caused by the introduction of this residue affects other residues in the R1-C1, R1-C4 and C1-C4 interfaces, which are involved in the quaternary structure
Quaternary structure
In biochemistry, quaternary structure is the arrangement of multiple folded protein or coiling protein molecules in a multi-subunit complex.-Description and examples:...
transition.

