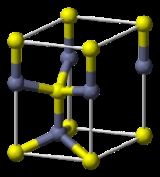
Wurtzite crystal structure
Encyclopedia
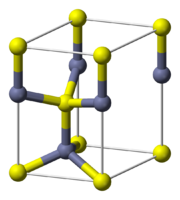
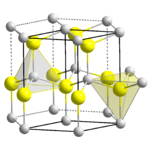

Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
, named after the mineral wurtzite
Wurtzite
Wurtzite is a zinc iron sulfide mineral a less frequently encountered mineral form of sphalerite. The iron content is variable up to eight percent. It is trimorphous with matraite and sphalerite....
, is a crystal structure for various binary compound
Binary compound
A binary compound is a chemical compound that contains exactly two different elements. Examples of binary ionic compounds include calcium chloride , sodium fluoride , and magnesium oxide , whilst examples of binary covalent compounds include water , carbon monoxide , and sulfur hexafluoride...
s. It is an example of a hexagonal crystal system
Hexagonal crystal system
In crystallography, the hexagonal crystal system is one of the 7 crystal systems, the hexagonal lattice system is one of the 7 lattice systems, and the hexagonal crystal family is one of the 6 crystal families...
.
Among the compounds that can take the wurtzite structure are wurtzite itself, AgI
Silver iodide
Silver iodide is a yellow, inorganic, photosensitive iodide of silver used in photography, in medicine as an antiseptic, and in rainmaking for cloud seeding.-Crystal structure:...
, ZnO
Zinc oxide
Zinc oxide is an inorganic compound with the formula ZnO. It is a white powder that is insoluble in water. The powder is widely used as an additive into numerous materials and products including plastics, ceramics, glass, cement, rubber , lubricants, paints, ointments, adhesives, sealants,...
, CdS
Cadmium sulfide
Cadmium sulfide is the inorganic compound with the formula CdS. Cadmium sulfide is a yellow solid. It occurs in nature with two different crystal structures as the rare minerals greenockite and hawleyite, but is more prevalent as an impurity substituent in the similarly structured zinc ores...
, CdSe
Cadmium selenide
Cadmium selenide is a solid, binary compound of cadmium and selenium. Common names for this compound are cadmium selenide, cadmium selenide, and cadmoselite ....
, α-SiC
Silicon carbide
Silicon carbide , also known as carborundum, is a compound of silicon and carbon with chemical formula SiC. It occurs in nature as the extremely rare mineral moissanite. Silicon carbide powder has been mass-produced since 1893 for use as an abrasive...
, GaN
Gallium(III) nitride
Gallium nitride is a binary III/V direct bandgap semiconductor commonly used in bright light-emitting diodes since the 1990s. The compound is a very hard material that has a Wurtzite crystal structure. Its wide band gap of 3.4 eV affords it special properties for applications in optoelectronic,...
, AlN
Aluminium nitride
Aluminium nitride is a nitride of aluminium. Its wurtzite phase is a wide band gap semiconductor material, giving it potential application for deep ultraviolet optoelectronics.-History:...
, and other semiconductors. In most of these compounds, wurtzite is not the favored form of the bulk crystal, but the structure can be favored in some nanocrystal forms of the material.
In materials with more than one crystal structure, the prefix "w-" is sometimes added to the empirical formula
Empirical formula
In chemistry, the empirical formula of a chemical compound is the simplest positive integer ratio of atoms of each element present in a compound. An empirical formula makes no reference to isomerism, structure, or absolute number of atoms. The empirical formula is used as standard for most ionic...
to denote the wurtzite crystal structure, as in w-BN.
Each of the two individual atom types forms a sublattice which is HCP-type
Close-packing
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement . Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occupied by spheres – that can be achieved by a regular lattice...
(short for "hexagonal close-pack"). When viewed altogether, the atomic positions are the same as in lonsdaleite
Lonsdaleite
Lonsdaleite , also called hexagonal diamond in reference to the crystal structure, is an allotrope of carbon with a hexagonal lattice. In nature, it forms when meteorites containing graphite strike the Earth. The great heat and stress of the impact transforms the graphite into diamond, but retains...
(hexagonal diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
). Each atom is tetrahedrally coordinated.
The wurtzite structure is non-centrosymmetric
Centrosymmetry
The term centrosymmetric, as generally used in crystallography, refers to a space group which contains an inversion center as one of its symmetry elements. In such a space group, for every point in the unit cell there is an indistinguishable point...
(i.e., lacks inversion symmetry). Due to this, wurtzite crystals can (and generally do) have properties such as piezoelectricity
Piezoelectricity
Piezoelectricity is the charge which accumulates in certain solid materials in response to applied mechanical stress. The word piezoelectricity means electricity resulting from pressure...
and pyroelectricity
Pyroelectricity
Pyroelectricity is the ability of certain materials to generate a temporary voltage when they are heated or cooled. The change in temperature modifies the positions of the atoms slightly within the crystal structure, such that the polarization of the material changes. This polarization change...
, which centrosymmetric crystals lack.

