Total organic carbon
Encyclopedia
Total organic carbon is the amount of carbon
bound in an organic compound
and is often used as a non-specific indicator of water quality
or cleanliness of pharmaceutical manufacturing equipment.
A typical analysis for TOC measures both the total carbon present as well as the so called "inorganic carbon" (IC), the latter representing the content of dissolved carbon dioxide and carbonic acid salts. Subtracting the inorganic carbon from the total carbon yields TOC. Another common variant of TOC analysis involves removing the IC portion first and then measuring the leftover carbon. This method involves purging an acid
ified sample with carbon-free air or nitrogen
prior to measurement, and so is more accurately called non-purgeable organic carbon (NPOC).
process. TOC in source waters comes from decaying natural organic matter (NOM) and from synthetic
sources. Humic acid
, fulvic acid, amine
s, and urea
are types of NOM. Detergents, pesticides, fertilizers, herbicides, industrial chemicals, and chlorinated
organics are examples of synthetic sources. Before source water is treated for disinfection, TOC provides an important role in quantifying the amount of NOM in the water source. In water treatment
facilities, source water is subject to reaction with chloride
containing disinfectants. When the raw water is chlorinated, active chlorine
compounds (Cl2, HOCl, ClO-) react with NOM to produce chlorinated disinfection byproducts (DBPs). Many researchers have determined that higher levels of NOM in source water during the disinfection process will increase the amount of carcinogen
s in the processed drinking water.
With passage of the U.S. Safe Drinking Water Act
in 1974, TOC analysis emerged as a rapid and accurate alternative to the classical but lengthy biological oxygen demand
(BOD) and chemical oxygen demand
(COD) tests traditionally reserved for assessing the pollution
potential of wastewater
s. Today, environmental agencies regulate the trace limits of DBPs in drinking water. Recently published analytical methods, such as United States Environmental Protection Agency
(EPA) method 415.3, support the Agency's Disinfectants and Disinfection Byproducts Rules, which regulate the amount of NOM to prevent the formation of DBPs in finished waters.
and distribution system materials. A relationship may exist between endotoxin
s, microbial
growth, and the development of biofilm
s on pipeline
walls and biofilm growth within pharmaceutical distribution systems. A correlation is believed to exist between TOC concentrations and the levels of endotoxins and microbes. Sustaining low TOC levels helps to control levels of endotoxins and microbes and thereby the development of biofilm growth. The United States Pharmacopoeia (USP), European Pharmacopoeia
(EP) and Japanese Pharmacopoeia (JP) recognize TOC as a required test for purified water and water for injection (WFI). For this reason, TOC has found acceptance as a process control attribute in the biotechnology
industry to monitor the performance of unit operations comprising purification and distribution systems. As many of these biotechnology operations include the preparation of medicines, the U.S. Food and Drug Administration (FDA) enacts numerous regulations to protect the health of the public and ensure the product quality is maintained. To make sure there is no cross-contamination
between product runs of different drugs, various cleaning procedures are performed. TOC concentration levels are used to track the success of these cleaning validation procedures especially clean-in-place
(CIP).
 Since all TOC analyzers only actually measure total carbon, TOC analysis always requires some accounting for the inorganic carbon that is always present. One analysis technique involves a two-stage process commonly referred to as TC-IC. It measures the amount of inorganic carbon (IC) evolved from an acidified aliquot
Since all TOC analyzers only actually measure total carbon, TOC analysis always requires some accounting for the inorganic carbon that is always present. One analysis technique involves a two-stage process commonly referred to as TC-IC. It measures the amount of inorganic carbon (IC) evolved from an acidified aliquot
of a sample and also the amount of total carbon (TC) present in the sample. TOC is calculated by subtraction of the IC value from the TC the sample. Another variant employs acidification of the sample to evolve carbon dioxide and measuring it as inorganic carbon (IC), then oxidizing
and measuring the remaining non-purgeable organic carbon (NPOC). This is called TIC-NPOC analysis. A more common method directly measures TOC in the sample by again acidifying the sample it to a pH
value of two or less to release the IC gas but in this case to the air not for measurement. The remaining non-purgeable CO2 gas (NPOC) contained in the liquid aliquot is then oxidized releasing the gases. These gases are then sent to the detector for measurement.
Whether the analysis of TOC is by TC-IC or NPOC methods, it may be broken into three main stages:
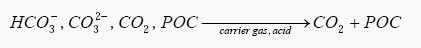
The first stage is acidification of the sample for the removal of the IC and POC gases. The release of these gases to the detector for measurement or to the air is dependent upon which type of analysis is of interest, the former for TC-IC and the latter for TOC (NPOC).
occurs in the following manner.
-rich atmosphere. All carbon present converts to carbon dioxide, flows through scrubber tubes
to remove interferences such as chlorine gas, and water vapor
, and the carbon dioxide is measured either by absorption into a strong base then weighed, or using an Infrared Detector
. Most modern analyzers use non-dispersive
infrared (NDIR) for detection of the carbon dioxide.
catalyst at 680 °C in an oxygen rich atmosphere. The concentration of carbon dioxide generated is measured with a non-dispersive infrared (NDIR) detector.
 Oxidation of the sample is complete after injection into the furnace, turning oxidizable material in the sample into gaseous form
Oxidation of the sample is complete after injection into the furnace, turning oxidizable material in the sample into gaseous form
. A carbon-free carrier gas transports the CO2, through a moisture
trap and halide
scrubbers to remove water vapor and halides from the gas stream before it reaches the detector. These substances can interfere with the detection of the CO2 gas. The HTCO method may be useful in those applications where difficult to oxidize compounds, or high molecular weight organics, are present as it provides almost complete oxidation of organics including solids and particulates small enough to be injected into the furnace. The major drawback of HTCO analysis is its unstable baseline resulting from the gradual accumulation of non-volatile
residues
within the combustion tube. These residues continuously change TOC background levels requiring continuous background correction. Because aqueous
samples are injected directly into a very hot, usually quartz
, furnace only small aliquots (less than 2 milliliters and usually less than 400 micro-liters) of sample can be handled making the methods less sensitive than chemical oxidation methods capable of digesting as much as 10 times more sample. Also, the salt content of the samples do not combust, and so therefore, gradually build a residue inside the combustion tube eventually clogging the catalyst resulting in poor peak shapes, and degraded accuracy or precision, unless appropriate maintenance procedures are followed. The catalyst should be regenerated or replaced as needed.
alone oxidizes the carbon within the sample to produce CO2. The UV oxidation method offers the most reliable, low maintenance method of analyzing TOC in ultra-pure waters.
compound. The mechanisms of the reactions are as follows:
The UV–chemical oxidation method offers a relatively low maintenance, high sensitivity method for a wide range of applications. However, there are oxidation limitations of this method. Limitations include the inaccuracies associated with the addition of any foreign substance into the analyte and samples with high amounts of particulates. Performing "System Blank" analysis, which is to analyze then subtract the amount of carbon contributed by the chemical additive, inaccuracies are lowered. However, analyses of levels below 200 ppb
TOC are still difficult.
formation as UV persulfate oxidation except uses heat to magnify the oxidizing power of persulfate. Chemical oxidation of carbon with a strong oxidizer, such as persulfate, is highly efficient, and unlike UV, is not susceptible to lower recoveries caused by turbidity
in samples. The analysis of system blanks, necessary in all chemical procedures, is especially necessary with heated persulfate TOC methods because the method is so sensitive that reagent
s cannot be prepared with carbon contents low enough to not be detected. Persulfate methods are used in the analysis of wastewater, drinking water, and pharmaceutical waters. When used in conjunction with sensitive NDIR detectors heated persulfate TOC instruments readily measure TOC at single digit parts per billion (ppb) up to hundreds of parts per million (ppm) depending on sample volumes.
, rather than relying on a measurement of a secondary, corrected effect, such as used in conductivity measurements.
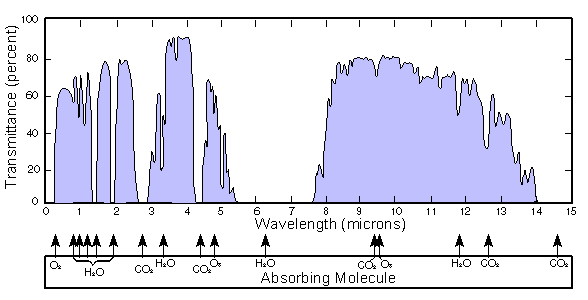
A traditional NDIR detector relies upon flow-through-cell technology, the oxidation product flows into and out of the detector continuously. A region of adsorption of infrared light specific to CO2, usually around 4.26 µm (2350 cm−1), is measured over time as the gas flows through the detector. The infrared absorption spectra of CO2 and other gases is shown in Figure 3. A second reference measurement that is non-specific to CO2 is also taken and the differential result correlates
to the CO2 concentration in the detector at that moment. As the gas continues to flow into and out of the detector cell the sum of the measurements results in a peak that is integrated
and correlated to the total CO2 concentration in the sample aliquot.
 Recent Advances in NDIR Technology
Recent Advances in NDIR Technology
A new advance of NDIR technology is Static Pressurized Concentration (SPC).
The exit valve of the NDIR is closed to allow the detector to become pressurized. Once the gases in the detector have reached equilibrium
, the concentration of the CO2 is analyzed. This pressurization of the sample gas stream in the NDIR, a patent-pending technique, allows for increased sensitivity and precision by measuring the entirety of the oxidation products of the sample in one reading, compared to flow-through cell technology. The output signal is proportional to the concentration of CO2 in the carrier gas, from the oxidation of the sample aliquot. UV/ Persulfate oxidation combined with NDIR detection provides good oxidation of organics, low instrument maintenance, good precision at ppb levels, relatively fast sample analysis time and easily accommodates multiple applications, including purified water (PW), water for injection (WFI), CIP, drinking water and ultra-pure water analyses.
reactor. Once the CO2 is formed, it is measured by a detector: either a conductivity cell (if the CO2 is aqueous) or a non-dispersive infrared cell (after purging the aqueous CO2 into the gaseous phase). Conductivity detection is only desirable in the lower TOC ranges in deionized waters, whereas NDIR detection excels in all TOC ranges. A variation described as Membrane Conductivity Detection can allow for measurement of TOC across a wide analytical range in both deionized and non-deionized water samples. Modern high-performance TOC instruments are capable of detecting carbon concentrations well below 1 µg/L (1 part per billion or ppb).
A total organic carbon analyzer determines the amount of carbon in a water sample. By acidifying the sample and flushing with nitrogen or helium the sample removes inorganic carbon, leaving only organic carbon sources for measurement. There are two types of analyzers. One uses combustion and the other chemical oxidation. This is used as a water purity test, as the presence of bacteria introduces organic carbon.
This is then sent to a detector for measurement. The other half of the sample is injected into a combustion chamber which is raised to between 600–700°C, some even up to 1200°C. Here, all the carbon reacts with oxygen, forming carbon dioxide. It's then flushed into a cooling chamber, and finally into the detector. Usually, the detector used is a non-dispersive infrared spectrophotometer. By finding the total inorganic carbon and subtracting it from the total carbon content, the amount of organic carbon is determined.
This method is often used in online applications because of its low maintenance requirements.
For example the online Biotector which is the most modern application of this method.
in oil exploration
. It is very important in detecting contaminants in drinking water, cooling water, water used in semiconductor manufacturing, and water for pharmaceutical use. Analysis may be made either as an online continuous measurement or a lab-based measurement.
TOC detection is an important measurement because of the effects it may have on the environment, human health, and manufacturing processes. TOC is a highly sensitive, non-specific measurement of all organics present in a sample. It, therefore, can be used to regulate the organic chemical discharge to the environment in a manufacturing plant. In addition, low TOC can confirm the absence of potentially harmful organic chemicals in water used to manufacture pharmaceutical products. TOC is also of interest in the field of potable water purification due to disinfection of byproducts. Inorganic carbon poses little to no threat.
Eq.1. Titrant normality equation:

where:
Eq. 2. Organic carbon percentage:

where:
We have milliequivalents as result of the difference between A and B, and they need to be converted to carbon milliequivalents in order to get the units we need, for that it is necessary to do the next operation:
Eq. 3

The 0.3 conversion factor has units of carbon grams and involves the constant to convert a fraction to percent units; hence equation 2 does not have the factor 100.
Walkey-Black constant for sediments. 75% is the mean recuperation of carbon in solids and sediments by using this method, that's why the final result has to be multiplied by 1.33 in order to get the real value, this constant is not used when determining carbon in KHP standard because almost all its carbon content is recovered.
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
bound in an organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
and is often used as a non-specific indicator of water quality
Water quality
Water quality is the physical, chemical and biological characteristics of water. It is a measure of the condition of water relative to the requirements of one or more biotic species and or to any human need or purpose. It is most frequently used by reference to a set of standards against which...
or cleanliness of pharmaceutical manufacturing equipment.
A typical analysis for TOC measures both the total carbon present as well as the so called "inorganic carbon" (IC), the latter representing the content of dissolved carbon dioxide and carbonic acid salts. Subtracting the inorganic carbon from the total carbon yields TOC. Another common variant of TOC analysis involves removing the IC portion first and then measuring the leftover carbon. This method involves purging an acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
ified sample with carbon-free air or nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
prior to measurement, and so is more accurately called non-purgeable organic carbon (NPOC).
Environmental
Since the early 1970s, TOC has been recognized as an analytic technique to measure water quality during the drinking water purificationWater purification
Water purification is the process of removing undesirable chemicals, materials, and biological contaminants from contaminated water. The goal is to produce water fit for a specific purpose...
process. TOC in source waters comes from decaying natural organic matter (NOM) and from synthetic
Chemical synthesis
In chemistry, chemical synthesis is purposeful execution of chemical reactions to get a product, or several products. This happens by physical and chemical manipulations usually involving one or more reactions...
sources. Humic acid
Humic acid
Humic acid is a principal component of humic substances, which are the major organic constituents of soil , peat, coal, many upland streams, dystrophic lakes, and ocean water. It is produced by biodegradation of dead organic matter...
, fulvic acid, amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s, and urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
are types of NOM. Detergents, pesticides, fertilizers, herbicides, industrial chemicals, and chlorinated
Chlorination
Chlorination is the process of adding the element chlorine to water as a method of water purification to make it fit for human consumption as drinking water...
organics are examples of synthetic sources. Before source water is treated for disinfection, TOC provides an important role in quantifying the amount of NOM in the water source. In water treatment
Water treatment
Water treatment describes those processes used to make water more acceptable for a desired end-use. These can include use as drinking water, industrial processes, medical and many other uses. The goal of all water treatment process is to remove existing contaminants in the water, or reduce the...
facilities, source water is subject to reaction with chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
containing disinfectants. When the raw water is chlorinated, active chlorine
Percent active chlorine
Percent active chlorine is a unit of concentration used for hypochlorite-based bleaches. One gram of a 100% active chlorine bleach has the same bleaching power as one gram of chlorine...
compounds (Cl2, HOCl, ClO-) react with NOM to produce chlorinated disinfection byproducts (DBPs). Many researchers have determined that higher levels of NOM in source water during the disinfection process will increase the amount of carcinogen
Carcinogen
A carcinogen is any substance, radionuclide, or radiation that is an agent directly involved in causing cancer. This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes...
s in the processed drinking water.
With passage of the U.S. Safe Drinking Water Act
Safe Drinking Water Act
The Safe Drinking Water Act is the principle federal law in the United States intended to ensure safe drinking water for the public. Pursuant to the act, the Environmental Protection Agency is required to set standards for drinking water quality and oversee all states, localities, and water...
in 1974, TOC analysis emerged as a rapid and accurate alternative to the classical but lengthy biological oxygen demand
Biochemical oxygen demand
Biochemical oxygen demand or B.O.D. is the amount of dissolved oxygen needed by aerobic biological organisms in a body of water to break down organic material present in a given water sample at certain temperature over a specific time period. The term also refers to a chemical procedure for...
(BOD) and chemical oxygen demand
Chemical oxygen demand
In environmental chemistry, the chemical oxygen demand test is commonly used to indirectly measure the amount of organic compounds in water. Most applications of COD determine the amount of organic pollutants found in surface water or wastewater, making COD a useful measure of water quality...
(COD) tests traditionally reserved for assessing the pollution
Water pollution
Water pollution is the contamination of water bodies . Water pollution occurs when pollutants are discharged directly or indirectly into water bodies without adequate treatment to remove harmful compounds....
potential of wastewater
Wastewater
Wastewater is any water that has been adversely affected in quality by anthropogenic influence. It comprises liquid waste discharged by domestic residences, commercial properties, industry, and/or agriculture and can encompass a wide range of potential contaminants and concentrations...
s. Today, environmental agencies regulate the trace limits of DBPs in drinking water. Recently published analytical methods, such as United States Environmental Protection Agency
United States Environmental Protection Agency
The U.S. Environmental Protection Agency is an agency of the federal government of the United States charged with protecting human health and the environment, by writing and enforcing regulations based on laws passed by Congress...
(EPA) method 415.3, support the Agency's Disinfectants and Disinfection Byproducts Rules, which regulate the amount of NOM to prevent the formation of DBPs in finished waters.
Pharmaceutical
Introduction of organic matter into water systems occurs not only from living organisms and from decaying matter in source water, but also from purificationWater purification
Water purification is the process of removing undesirable chemicals, materials, and biological contaminants from contaminated water. The goal is to produce water fit for a specific purpose...
and distribution system materials. A relationship may exist between endotoxin
Endotoxin
Endotoxins are toxins associated with some Gram-negative bacteria. An "endotoxin" is a toxin that is a structural molecule of the bacteria that is recognized by the immune system.-Gram negative:...
s, microbial
Microorganism
A microorganism or microbe is a microscopic organism that comprises either a single cell , cell clusters, or no cell at all...
growth, and the development of biofilm
Biofilm
A biofilm is an aggregate of microorganisms in which cells adhere to each other on a surface. These adherent cells are frequently embedded within a self-produced matrix of extracellular polymeric substance...
s on pipeline
Pipeline transport
Pipeline transport is the transportation of goods through a pipe. Most commonly, liquids and gases are sent, but pneumatic tubes that transport solid capsules using compressed air are also used....
walls and biofilm growth within pharmaceutical distribution systems. A correlation is believed to exist between TOC concentrations and the levels of endotoxins and microbes. Sustaining low TOC levels helps to control levels of endotoxins and microbes and thereby the development of biofilm growth. The United States Pharmacopoeia (USP), European Pharmacopoeia
European Pharmacopoeia
The European Pharmacopoeia of the Council of Europe is a pharmacopoeia, listing a wide range of active substances and excipients used to prepare pharmaceutical products in Europe...
(EP) and Japanese Pharmacopoeia (JP) recognize TOC as a required test for purified water and water for injection (WFI). For this reason, TOC has found acceptance as a process control attribute in the biotechnology
Biotechnology
Biotechnology is a field of applied biology that involves the use of living organisms and bioprocesses in engineering, technology, medicine and other fields requiring bioproducts. Biotechnology also utilizes these products for manufacturing purpose...
industry to monitor the performance of unit operations comprising purification and distribution systems. As many of these biotechnology operations include the preparation of medicines, the U.S. Food and Drug Administration (FDA) enacts numerous regulations to protect the health of the public and ensure the product quality is maintained. To make sure there is no cross-contamination
Foodborne illness
Foodborne illness is any illness resulting from the consumption of contaminated food, pathogenic bacteria, viruses, or parasites that contaminate food, as well as chemical or natural toxins such as poisonous mushrooms.-Causes:Foodborne illness usually arises from improper handling, preparation, or...
between product runs of different drugs, various cleaning procedures are performed. TOC concentration levels are used to track the success of these cleaning validation procedures especially clean-in-place
Clean-in-place
Clean-in-Place is a method of cleaning the interior surfaces of pipes, vessels, process equipment, filters and associated fittings, without disassembly....
(CIP).
Measurement
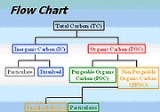
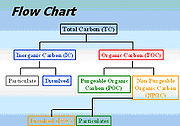
To understand the analysis process better, some key basic terminologies should be understood and their relationships to one another (Figure 1).- Total Carbon (TC) – all the carbon in the sample, including both inorganic and organic carbon
- Total Inorganic Carbon (TIC) – often referred to as inorganic carbon (IC), carbonateCarbonateIn chemistry, a carbonate is a salt of carbonic acid, characterized by the presence of the carbonate ion, . The name may also mean an ester of carbonic acid, an organic compound containing the carbonate group C2....
, bicarbonateBicarbonateIn inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
, and dissolvedSolvationSolvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
carbon dioxideCarbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(CO2). - Total Organic Carbon (TOC) – material derived from decaying vegetation, bacterial growth, and metabolicMetabolismMetabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
activities of living organisms or chemicals. - Non-Purgeable Organic Carbon (NPOC) – commonly referred to as TOC; organic carbon remaining in an acidified sample after purging the sample with gas.
- Purgeable (volatile) Organic Carbon (VOC) – organic carbon that has been removed from a neutralPHIn chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
, or acidified sample by purging with an inert gasInert gasAn inert gas is a non-reactive gas used during chemical synthesis, chemical analysis, or preservation of reactive materials. Inert gases are selected for specific settings for which they are functionally inert since the cost of the gas and the cost of purifying the gas are usually a consideration...
. These are the same compounds referred to as Volatile Organic CompoundsVolatile organic compoundVolatile organic compounds are organic chemicals that have a high vapor pressure at ordinary, room-temperature conditions. Their high vapor pressure results from a low boiling point, which causes large numbers of molecules to evaporate or sublimate from the liquid or solid form of the compound and...
(VOC) and usually determined by Purge and Trap Gas ChromatographyGas chromatography-mass spectrometryGas chromatography–mass spectrometry is a method that combines the features of gas-liquid chromatography and mass spectrometry to identify different substances within a test sample. Applications of GC-MS include drug detection, fire investigation, environmental analysis, explosives investigation,...
. - Dissolved Organic Carbon (DOC) – organic carbon remaining in a sample after filtering the sample, typically using a 0.45 micrometerMicrometreA micrometer , is by definition 1×10-6 of a meter .In plain English, it means one-millionth of a meter . Its unit symbol in the International System of Units is μm...
filter. - Suspended Organic Carbon – also called particulate organic carbon (POC); the carbon in particulate form that is too large to pass through a filter.

Aliquot
Aliquot may refer to:In mathematics:*Aliquot part, a proper divisor of an integer*Aliquot sum, the sum of the aliquot parts of an integer*Aliquot sequence, a sequence of integers in which each number is the aliquot sum of the previous numberIn music:...
of a sample and also the amount of total carbon (TC) present in the sample. TOC is calculated by subtraction of the IC value from the TC the sample. Another variant employs acidification of the sample to evolve carbon dioxide and measuring it as inorganic carbon (IC), then oxidizing
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
and measuring the remaining non-purgeable organic carbon (NPOC). This is called TIC-NPOC analysis. A more common method directly measures TOC in the sample by again acidifying the sample it to a pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
value of two or less to release the IC gas but in this case to the air not for measurement. The remaining non-purgeable CO2 gas (NPOC) contained in the liquid aliquot is then oxidized releasing the gases. These gases are then sent to the detector for measurement.
Whether the analysis of TOC is by TC-IC or NPOC methods, it may be broken into three main stages:
- Acidification
- Oxidation
- Detection and Quantification
The first stage is acidification of the sample for the removal of the IC and POC gases. The release of these gases to the detector for measurement or to the air is dependent upon which type of analysis is of interest, the former for TC-IC and the latter for TOC (NPOC).
Acidification
The removal and venting of IC and POC gases from the liquid sample by acidification and spargingSparging (chemistry)
In chemistry, sparging, also known as gas flushing in metallurgy, is a technique which involves bubbling a chemically inert gas, such as nitrogen, argon, or helium, through a liquid. This can be used to remove dissolved gases In chemistry, sparging, also known as gas flushing in metallurgy, is a...
occurs in the following manner.

Oxidation
The second stage is the oxidation of the carbon in the remaining sample in the form of carbon dioxide (CO2) and other gases. Modern TOC analyzers perform this oxidation step by several processes:- High Temperature CombustionCombustionCombustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
- High temperature catalyticCatalysisCatalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
(HTCO) oxidation - Photo-oxidationPhoto-oxidation of polymersPhoto-oxidation is the degradation of a polymer surface in the presence of oxygen or ozone. The effect is facilitated by radiant energy such as UV or artificial light. This process is the most significant factor in weathering of polymers. Photo-oxidation is a chemical change that reduces the...
alone - Thermo-chemical oxidation
- Photo-chemicalPhotochemistryPhotochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.-Principles:Light is a type of...
oxidation - ElectrolyticElectrolyteIn chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
Oxidation
High temperature combustion
Prepared samples are combusted at 1,350 °C in an oxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
-rich atmosphere. All carbon present converts to carbon dioxide, flows through scrubber tubes
Carbon dioxide scrubber
A carbon dioxide scrubber is a device which absorbs carbon dioxide . It is used to treat exhaust gases from industrial plants or from exhaled air in life support systems such as rebreathers or in spacecraft, submersible craft or airtight chambers...
to remove interferences such as chlorine gas, and water vapor
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
, and the carbon dioxide is measured either by absorption into a strong base then weighed, or using an Infrared Detector
Infrared detector
An infrared detector is a photodetector that reacts to infrared radiation. The two main types of detectors are thermal and photonic.The thermal effects of the incident IR radiation can be followed through many temperature dependent phenomena....
. Most modern analyzers use non-dispersive
Dispersion (optics)
In optics, dispersion is the phenomenon in which the phase velocity of a wave depends on its frequency, or alternatively when the group velocity depends on the frequency.Media having such a property are termed dispersive media...
infrared (NDIR) for detection of the carbon dioxide.
High temperature catalytic oxidation
A manual or automated process injects the sample onto a platinumPlatinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
catalyst at 680 °C in an oxygen rich atmosphere. The concentration of carbon dioxide generated is measured with a non-dispersive infrared (NDIR) detector.

Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
. A carbon-free carrier gas transports the CO2, through a moisture
Moisture
Humidity is the amount of moisture the air can hold before it rains. Moisture refers to the presence of a liquid, especially water, often in trace amounts...
trap and halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
scrubbers to remove water vapor and halides from the gas stream before it reaches the detector. These substances can interfere with the detection of the CO2 gas. The HTCO method may be useful in those applications where difficult to oxidize compounds, or high molecular weight organics, are present as it provides almost complete oxidation of organics including solids and particulates small enough to be injected into the furnace. The major drawback of HTCO analysis is its unstable baseline resulting from the gradual accumulation of non-volatile
Volatility (chemistry)
In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor pressure. At a given temperature, a substance with higher vapor pressure vaporizes more readily than a substance with a lower vapor pressure.The term is primarily...
residues
Residue (chemistry)
In chemistry, residue is the material remaining after a distillation or an evaporation, or to a portion of a larger molecule, such as a methyl group. It may also refer to the undesired byproducts of a reaction....
within the combustion tube. These residues continuously change TOC background levels requiring continuous background correction. Because aqueous
Aqueous solution
An aqueous solution is a solution in which the solvent is water. It is usually shown in chemical equations by appending aq to the relevant formula, such as NaCl. The word aqueous means pertaining to, related to, similar to, or dissolved in water...
samples are injected directly into a very hot, usually quartz
Quartz
Quartz is the second-most-abundant mineral in the Earth's continental crust, after feldspar. It is made up of a continuous framework of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall formula SiO2. There are many different varieties of quartz,...
, furnace only small aliquots (less than 2 milliliters and usually less than 400 micro-liters) of sample can be handled making the methods less sensitive than chemical oxidation methods capable of digesting as much as 10 times more sample. Also, the salt content of the samples do not combust, and so therefore, gradually build a residue inside the combustion tube eventually clogging the catalyst resulting in poor peak shapes, and degraded accuracy or precision, unless appropriate maintenance procedures are followed. The catalyst should be regenerated or replaced as needed.
Photo-Oxidation (UV Light)
In this oxidation scheme, ultra-violet lightUltraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
alone oxidizes the carbon within the sample to produce CO2. The UV oxidation method offers the most reliable, low maintenance method of analyzing TOC in ultra-pure waters.
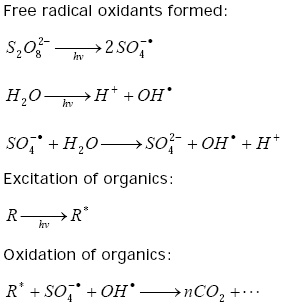
The UV/Chemical (Persulfate) Oxidation
Like the photo-oxidation method, UV light is the oxidizer but the oxidation power of the reaction is magnified by the addition of a chemical oxidizer, which is usually a persulfatePersulfate
The term persulfate refers to ions or compounds with more oxygen than normal sulfates.These do not have sulfur in a different oxidation state; rather, they contain peroxide units, where two oxygens take the place of one in a normal sulfate; the oxygen atoms are in oxidation state −1.The main forms...
compound. The mechanisms of the reactions are as follows:

The UV–chemical oxidation method offers a relatively low maintenance, high sensitivity method for a wide range of applications. However, there are oxidation limitations of this method. Limitations include the inaccuracies associated with the addition of any foreign substance into the analyte and samples with high amounts of particulates. Performing "System Blank" analysis, which is to analyze then subtract the amount of carbon contributed by the chemical additive, inaccuracies are lowered. However, analyses of levels below 200 ppb
Parts-per notation
In science and engineering, the parts-per notation is a set of pseudo units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, they are pure numbers with no associated units of measurement...
TOC are still difficult.
Thermo-Chemical (Persulfate) Oxidation
Also known as heated persulfate, the method utilizes the same free radicalRadical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
formation as UV persulfate oxidation except uses heat to magnify the oxidizing power of persulfate. Chemical oxidation of carbon with a strong oxidizer, such as persulfate, is highly efficient, and unlike UV, is not susceptible to lower recoveries caused by turbidity
Turbidity
Turbidity is the cloudiness or haziness of a fluid caused by individual particles that are generally invisible to the naked eye, similar to smoke in air. The measurement of turbidity is a key test of water quality....
in samples. The analysis of system blanks, necessary in all chemical procedures, is especially necessary with heated persulfate TOC methods because the method is so sensitive that reagent
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
s cannot be prepared with carbon contents low enough to not be detected. Persulfate methods are used in the analysis of wastewater, drinking water, and pharmaceutical waters. When used in conjunction with sensitive NDIR detectors heated persulfate TOC instruments readily measure TOC at single digit parts per billion (ppb) up to hundreds of parts per million (ppm) depending on sample volumes.
Detection and quantification
Accurate detection and quantification are the most vital components of the TOC analysis process. Conductivity and non-dispersive infrared (NDIR) are the two common detection methods used in modern TOC analyzers.Conductivity
There are two types of conductivity detectors, direct and membrane. Direct conductivity provides an inexpensive and simple means of measuring CO2. This method has good oxidation of organics, uses no carrier gas, is good at the parts per billion (ppb) ranges, but has a very limited analytical range. Membrane conductivity relies upon the same technology as direct conductivity. Although it is more robust than direct conductivity, it suffers from slow analysis time. Both methods analyze sample conductivity before and after oxidization, attributing this differential measurement to the TOC of the sample. During the sample oxidization phase, CO2 (directly related to the TOC in the sample) and other gases are formed. The dissolved CO2 forms a weak acid, thereby changing the conductivity of the original sample proportionately to the TOC in the sample. Conductivity analyses assume that only CO2 is present within the solution. As long as this holds true, then the TOC calculation by this differential measurement is valid. However, depending on the chemical species present in the sample and their individual products of oxidation, they may present either a positive or a negative interference to the actual TOC value, resulting in analytical error. Some of the interfering chemical species include Cl-, HCO3-, SO32-, SO2-, ClO2-, and H+. Small changes in pH and temperature fluctuations also contribute to inaccuracy. Membrane conductivity analyzers have tried to improve upon the direct conductivity approach by incorporating the use of hydrophobic gas permeation membranes to allow a more “selective” passage of the dissolved CO2 gas. While this has solved certain problems, membranes have their own particular limitations, such as with true selectivity, clogging and, more undetectably, they provide secondary sites for other chemical reactions, which are prone to display “false negatives,” a condition far more severe than “false positives” in critical applications. Micro leaks, flow problems, dead spots, microbial growth (blockage) are also potential problems. Most disconcerting is the inability of membrane methods to recover to operational performance after an overload or “spill” condition arises to over range the instrument, often taking hours before returning to reliable service and recalibration, just when accuracy of TOC analysis is most critical to operators for quality control.Non-dispersive infrared (NDIR)
The non-dispersive infrared analysis (NDIR) method offers the only practical interference-free method for detecting CO2 in TOC analysis. The principal advantage of using NDIR is that it directly and specifically measures the CO2 generated by oxidation of the organic carbon in the oxidation reactorChemical reactor
In chemical engineering, chemical reactors are vessels designed to contain chemical reactions. The design of a chemical reactor deals with multiple aspects of chemical engineering. Chemical engineers design reactors to maximize net present value for the given reaction...
, rather than relying on a measurement of a secondary, corrected effect, such as used in conductivity measurements.
A traditional NDIR detector relies upon flow-through-cell technology, the oxidation product flows into and out of the detector continuously. A region of adsorption of infrared light specific to CO2, usually around 4.26 µm (2350 cm−1), is measured over time as the gas flows through the detector. The infrared absorption spectra of CO2 and other gases is shown in Figure 3. A second reference measurement that is non-specific to CO2 is also taken and the differential result correlates
Correlation
In statistics, dependence refers to any statistical relationship between two random variables or two sets of data. Correlation refers to any of a broad class of statistical relationships involving dependence....
to the CO2 concentration in the detector at that moment. As the gas continues to flow into and out of the detector cell the sum of the measurements results in a peak that is integrated
Integral
Integration is an important concept in mathematics and, together with its inverse, differentiation, is one of the two main operations in calculus...
and correlated to the total CO2 concentration in the sample aliquot.

A new advance of NDIR technology is Static Pressurized Concentration (SPC).
The exit valve of the NDIR is closed to allow the detector to become pressurized. Once the gases in the detector have reached equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
, the concentration of the CO2 is analyzed. This pressurization of the sample gas stream in the NDIR, a patent-pending technique, allows for increased sensitivity and precision by measuring the entirety of the oxidation products of the sample in one reading, compared to flow-through cell technology. The output signal is proportional to the concentration of CO2 in the carrier gas, from the oxidation of the sample aliquot. UV/ Persulfate oxidation combined with NDIR detection provides good oxidation of organics, low instrument maintenance, good precision at ppb levels, relatively fast sample analysis time and easily accommodates multiple applications, including purified water (PW), water for injection (WFI), CIP, drinking water and ultra-pure water analyses.
Analyzers
Virtually all TOC analyzers measure the CO2 formed when organic carbon is oxidized and/or when inorganic carbon is acidified. Oxidation is performed either through Pt-catalyzed combustion, by heated persulfate, or with a UV/persulfateSodium persulfate
Sodium persulfate is a chemical compound. It is a strong oxidizer. It is a severe irritant of skin, eyes, and respiratory system. It is almost non-hygroscopic and has particularly good ability to be stored for long time. It is easy and safe to handle...
reactor. Once the CO2 is formed, it is measured by a detector: either a conductivity cell (if the CO2 is aqueous) or a non-dispersive infrared cell (after purging the aqueous CO2 into the gaseous phase). Conductivity detection is only desirable in the lower TOC ranges in deionized waters, whereas NDIR detection excels in all TOC ranges. A variation described as Membrane Conductivity Detection can allow for measurement of TOC across a wide analytical range in both deionized and non-deionized water samples. Modern high-performance TOC instruments are capable of detecting carbon concentrations well below 1 µg/L (1 part per billion or ppb).
A total organic carbon analyzer determines the amount of carbon in a water sample. By acidifying the sample and flushing with nitrogen or helium the sample removes inorganic carbon, leaving only organic carbon sources for measurement. There are two types of analyzers. One uses combustion and the other chemical oxidation. This is used as a water purity test, as the presence of bacteria introduces organic carbon.
Analyzer Field Testing and Reports
A non-profit research and testing organization, the Instrumentation Testing Association (ITA) offers a report that provides results of field testing online TOC analyzers in an industrial wastewater application. Gulf Coast Waste Disposal Authority (GCWDA), Bayport Industrial Wastewater Treatment Plant in Pasadena, Texas sponsored and conducted this test in 2011. The GCWDA Bayport facility treats approximately 30 mgd of industrial waste received from approximately 65 customers (primarily petrochemical). Field tests consisted of operating online TOC analyzers at the influent of the Bayport facility in which TOC concentrations can range from 490 to 1020 mg/L with an average of 870 mg/L. GCWDA conducts approximately 102 TOC analyses in their laboratory per day at their Bayport treatment facility and use TOC measurements for process control and billing purposes. GCWDA plans to use online TOC analyzers for process control, detecting influent slug loads from industries and to potentially use online TOC analyzers to detect and monitor volatiles of the incoming stream. Field tests were conducted for a period of 90-days and used laboratory conformance measurements once per day to compare with analyzer output to demonstrate the instrument's overall accuracy when subjected to many simultaneously changing parameters as experienced in real-time monitoring conditions. Field test results can provide information regarding instrument design, operation and maintenance requirements which influence the performance of the instruments in field applications. The field test report includes evaluations of online TOC analyzers utilizing the following technologies: High Temperature Combustion (HTC), High Temperature Catalytic/Combustion Oxidation (HTCO), Supercritical Water Oxidation (SCWO), and Two-Stage Advanced Oxidation (TSAO).Combustion
In a combustion analyzer, half the sample is injected into a chamber where it is acidified, usually with phosphoric acid, to turn all of the inorganic carbon into carbon dioxide as per the following reaction:- CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3- ↔ 2H+ + CO32-
This is then sent to a detector for measurement. The other half of the sample is injected into a combustion chamber which is raised to between 600–700°C, some even up to 1200°C. Here, all the carbon reacts with oxygen, forming carbon dioxide. It's then flushed into a cooling chamber, and finally into the detector. Usually, the detector used is a non-dispersive infrared spectrophotometer. By finding the total inorganic carbon and subtracting it from the total carbon content, the amount of organic carbon is determined.
Chemical oxidation
Chemical oxidation analyzers inject the sample into a chamber with phosphoric acid followed by persulfate. The analysis is separated into two steps. One removes inorganic carbon by acidification and purging. After removal of inorganic carbon persulfate is added and the sample is either heated or bombarded with UV light from a mercury vapor lamp. Free radicals form from the persulfate and react with any carbon available to form carbon dioxide. The carbon from both determination (steps) is either run through membranes which measure the conductivity changes that result from the presence of varying amounts of carbon dioxide, or purged into and detected by a sensitive NDIR detector. Same as the combustion analyzer, the total carbon formed minus the inorganic carbon gives a good estimate of the total organic carbon in the sample.This method is often used in online applications because of its low maintenance requirements.
For example the online Biotector which is the most modern application of this method.
Applications
TOC is the first chemical analysis to be carried out on potential petroleum source rockSource rock
In petroleum geology, source rock refers to rocks from which hydrocarbons have been generated or are capable of being generated. They form one of the necessary elements of a working petroleum system. They are organic-rich sediments that may have been deposited in a variety of environments including...
in oil exploration
Oil exploration
Hydrocarbon exploration is the search by petroleum geologists and geophysicists for hydrocarbon deposits beneath the Earth's surface, such as oil and natural gas...
. It is very important in detecting contaminants in drinking water, cooling water, water used in semiconductor manufacturing, and water for pharmaceutical use. Analysis may be made either as an online continuous measurement or a lab-based measurement.
TOC detection is an important measurement because of the effects it may have on the environment, human health, and manufacturing processes. TOC is a highly sensitive, non-specific measurement of all organics present in a sample. It, therefore, can be used to regulate the organic chemical discharge to the environment in a manufacturing plant. In addition, low TOC can confirm the absence of potentially harmful organic chemicals in water used to manufacture pharmaceutical products. TOC is also of interest in the field of potable water purification due to disinfection of byproducts. Inorganic carbon poses little to no threat.
Calculation
In the Total Organic Carbon determination to obtain the percent carbon content from soil, first we have to standardize the titrant solution (FeSO4•7H2O) before the sample analysis are made, as result we obtain some data which have to be reduced in order to obtain the results we need, and to do this, we use the next equations:Eq.1. Titrant normality equation:

where:
N1: K2Cr2O7 normality
V1: K2Cr2O7 volume (mL)
V2: FeSO4 volume (mL)
Eq. 2. Organic carbon percentage:

where:
A: meq K2Cr2O7 = (mL K2Cr2O7 x N K2Cr2O7)
B: meq FeSO4•7H2O = (mL FeSO4•7H2O x N FeSO4•7H2O)
C: grams of sample
0.3: Conversion factor to carbon weight.
We have milliequivalents as result of the difference between A and B, and they need to be converted to carbon milliequivalents in order to get the units we need, for that it is necessary to do the next operation:
Eq. 3

The 0.3 conversion factor has units of carbon grams and involves the constant to convert a fraction to percent units; hence equation 2 does not have the factor 100.
Walkey-Black constant for sediments. 75% is the mean recuperation of carbon in solids and sediments by using this method, that's why the final result has to be multiplied by 1.33 in order to get the real value, this constant is not used when determining carbon in KHP standard because almost all its carbon content is recovered.

