
Thiosulfate sulfurtransferase
Encyclopedia
In enzymology, a thiosulfate sulfurtransferase is an enzyme
that catalyzes
the chemical reaction
Thus, the two substrates
of this enzyme are thiosulfate
and cyanide
, whereas its two products
are sulfite
and thiocyanate
.
This enzyme belongs to the family of transferase
s, specifically the sulfurtransferases, which transfer sulfur-containing groups. The systematic name of this enzyme class is thiosulfate:cyanide sulfurtransferase. Other names in common use include thiosulfate cyanide transsulfurase, thiosulfate thiotransferase, rhodanese, and rhodanase.
have been solved for this class of enzymes, with PDB
accession codes , , , , , , , , , , and .
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- thiosulfate + cyanide
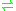 sulfite + thiocyanate
sulfite + thiocyanate
Thus, the two substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are thiosulfate
Thiosulfate
Thiosulfate is an oxyanion of sulfur. The prefix thio indicates that thiosulfate ion is a sulfate ion with one oxygen replaced by a sulfur. Thiosulfate occurs naturally and is produced by certain biochemical processes...
and cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
, whereas its two products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are sulfite
Sulfite
Sulfites are compounds that contain the sulfite ion SO. The sulfite ion is the conjugate base of bisulfite. Although the acid itself is elusive, its salts are widely used.-Structure:...
and thiocyanate
Thiocyanate
Thiocyanate is the anion [SCN]−. It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Organic compounds containing the functional group SCN are also called thiocyanates...
.
This enzyme belongs to the family of transferase
Transferase
In biochemistry, a transferase is an enzyme that catalyzes the transfer of a functional group from one molecule to another . For example, an enzyme that catalyzed this reaction would be a transferase:In this example, A would be the donor, and B would be the acceptor...
s, specifically the sulfurtransferases, which transfer sulfur-containing groups. The systematic name of this enzyme class is thiosulfate:cyanide sulfurtransferase. Other names in common use include thiosulfate cyanide transsulfurase, thiosulfate thiotransferase, rhodanese, and rhodanase.
Structural studies
As of late 2007, 11 structuresTertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
have been solved for this class of enzymes, with PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession codes , , , , , , , , , , and .

