
Thiolase
Encyclopedia
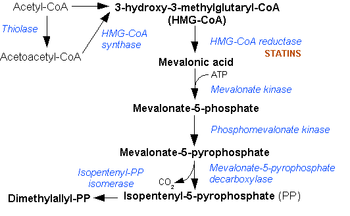
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
to acetoacetyl CoA in the mevalonate pathway.
Thiolases are ubiquitous enzymes that have key roles in many vital biochemical pathways, including the beta oxidation
Beta oxidation
Beta oxidation is the process by which fatty acids, in the form of Acyl-CoA molecules, are broken down in mitochondria and/or in peroxisomes to generate Acetyl-CoA, the entry molecule for the Citric Acid cycle....
pathway of fatty acid degradation and various biosynthetic pathways. Members of the thiolase family can be divided into two broad categories: degradative thiolases (EC 2.3.1.16) and biosynthetic thiolases (EC 2.3.1.9). These two different types of thiolase are found both in eukaryotes and in prokaryotes: acetoacetyl-CoA thiolase (EC:2.3.1.9) and 3-ketoacyl-CoA thiolase
Acetyl-CoA C-acyltransferase
The final step of beta oxidation is the cleavage of 3-ketoacyl CoA by the thiol group of another molecule of CoA. This reaction is catalyzed by Acetyl-CoA C-acyltransferase...
(EC:2.3.1.16). 3-ketoacyl-CoA thiolase (also called thiolase I) has a broad chain-length specificity for its substrates and is involved in degradative pathways such as fatty acid beta-oxidation. Acetoacetyl-CoA thiolase (also called thiolase II) is specific for the thiolysis of acetoacetyl-CoA and involved in biosynthetic pathways such as poly beta-hydroxybutyric acid synthesis or steroid
Steroid
A steroid is a type of organic compound that contains a characteristic arrangement of four cycloalkane rings that are joined to each other. Examples of steroids include the dietary fat cholesterol, the sex hormones estradiol and testosterone, and the anti-inflammatory drug dexamethasone.The core...
biogenesis.
The formation of a carbon–carbon bond is a key step in the biosynthetic pathways by which fatty acids and polyketide
Polyketide
Polyketides are secondary metabolites from bacteria, fungi, plants, and animals. Polyketides are usually biosynthesized through the decarboxylative condensation of malonyl-CoA derived extender units in a similar process to fatty acid synthesis...
are made. The thiolase superfamily enzymes catalyse the carbon–carbon-bond formation via a thioester-dependent Claisen condensation
Claisen condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone...
reaction mechanism.
Function
Thiolases are a familyProtein family
A protein family is a group of evolutionarily-related proteins, and is often nearly synonymous with gene family. The term protein family should not be confused with family as it is used in taxonomy....
of evolutionarily related enzymes. Two different types of thiolase are found both in eukaryotes and in prokaryotes: acetoacetyl-CoA thiolase
Acetyl-CoA C-acetyltransferase
In enzymology, an acetyl-CoA C-acetyltransferase is an enzyme that catalyzes the chemical reactionHence, this enzyme has one substrate, acetyl-CoA, and two products, CoA and acetoacetyl-CoA....
and 3-ketoacyl-CoA thiolase
Acetyl-CoA C-acyltransferase
The final step of beta oxidation is the cleavage of 3-ketoacyl CoA by the thiol group of another molecule of CoA. This reaction is catalyzed by Acetyl-CoA C-acyltransferase...
. 3-ketoacyl-CoA thiolase (also called thiolase I) has a broad chain-length specificity for its substrates and is involved in degradative pathways such as fatty acid beta-oxidation. Acetoacetyl-CoA thiolase (also called thiolase II) is specific for the thiolysis of acetoacetyl-CoA and involved in biosynthetic pathways such as poly beta-hydroxybutyrate synthesis or steroid biogenesis.
In eukaryotes, there are two forms of 3-ketoacyl-CoA thiolase: one located in the mitochondrion and the other in peroxisomes.
There are two conserved cysteine residues important for thiolase activity. The first located in the N-terminal section of the enzymes is involved in the formation of an acyl-enzyme intermediate; the second located at the C-terminal extremity is the active site base involved in deprotonation in the condensation reaction.
Isozymes
| EC number | Name | Alternate name | Isozymes | Subcellular distribution |
|---|---|---|---|---|
| Acetyl-CoA C-acetyltransferase Acetyl-CoA C-acetyltransferase In enzymology, an acetyl-CoA C-acetyltransferase is an enzyme that catalyzes the chemical reactionHence, this enzyme has one substrate, acetyl-CoA, and two products, CoA and acetoacetyl-CoA.... |
thiolase II; Acetoacetyl-CoA thiolase |
ACAT1 ACAT1 Acetyl-CoA acetyltransferase, mitochondrial also known as acetoacetyl-CoA thiolase, is an enzyme that in humans is encoded by the ACAT1 gene.... |
mitochondrial | |
| ACAT2 ACAT2 Acetyl-CoA acetyltransferase, cytosolic, also known as cytosolic acetoacetyl-CoA thiolase, is an enzyme that in humans is encoded by the ACAT2 gene that is responsible for the synthesis of cholesteryl esters which are part of lipoproteins containing apoB.Acetyl-Coenzyme A acetyltransferase 2 is... |
cytosolic | |||
| Acetyl-CoA C-acyltransferase Acetyl-CoA C-acyltransferase The final step of beta oxidation is the cleavage of 3-ketoacyl CoA by the thiol group of another molecule of CoA. This reaction is catalyzed by Acetyl-CoA C-acyltransferase... |
thiolase I; 3-Ketoacyl-CoA thiolase; β-Ketothiolase |
ACAA1 ACAA1 3-Ketoacyl-CoA thiolase, peroxisomal also known as acetyl-Coenzyme A acyltransferase 1 is an enzyme that in humans is encoded by the ACAA1 gene.Acetyl-Coenzyme A acyltransferase 1 is a acetyl-CoA C-acyltransferase enzyme.- Function :... |
peroxisomal | |
| ACAA2 ACAA2 3-Ketoacyl-CoA thiolase, mitochondrial also known as acetyl-Coenzyme A acyltransferase 2 is an enzyme that in humans is encoded by the ACAA2 gene.Acetyl-Coenzyme A acyltransferase 2 is a acetyl-CoA C-acyltransferase enzyme.- Function :... |
mitochondrial | |||
| HADHB HADHB Trifunctional enzyme subunit beta, mitochondrial also known as 3-ketoacyl-CoA thiolase, acetyl-CoA acyltransferase, or beta-ketothiolase is an enzyme that in humans is encoded by the HADHB gene.... |
mitochondrial | |||
| Propionyl-CoA C2-trimethyltridecanoyltransferase Propionyl-CoA C2-trimethyltridecanoyltransferase In enzymology, a propionyl-CoA C2-trimethyltridecanoyltransferase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are 4,8,12-trimethyltridecanoyl-CoA and propanoyl-CoA, whereas its two products are 3-oxopristanoyl-CoA and CoA.This enzyme belongs to the... |
3-Oxopristanoyl-CoA thiolase | |||
| 3-Oxoadipyl-CoA thiolase 3-oxoadipyl-CoA thiolase In enzymology, a 3-oxoadipyl-CoA thiolase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are succinyl-CoA and acetyl-CoA, whereas its two products are CoA and 3-oxoadipyl-CoA.... |
β-Ketoadipyl-CoA thiolase | |||
| Propanoyl-CoA C-acyltransferase Propanoyl-CoA C-acyltransferase In enzymology, a propanoyl-CoA C-acyltransferase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are 3alpha,7alpha,12alpha-trihydroxy-5beta-cholanoyl-CoA and propanoyl-CoA, whereas its two products are CoA and... |
Peroxisomal thiolase 2 | SCP2 SCP2 Non-specific lipid-transfer protein also known as sterol carrier protein 2 or propanoyl-CoA C-acyltransferase is a protein that in humans is encoded by the SCP2 gene.- Function :... |
peroxisomal/cytosolic |
Mammalian nonspecific lipid-transfer protein (nsL-TP) (also known as sterol carrier protein 2
SCP2
Non-specific lipid-transfer protein also known as sterol carrier protein 2 or propanoyl-CoA C-acyltransferase is a protein that in humans is encoded by the SCP2 gene.- Function :...
) is a protein which seems to exist in two different forms: a 14 Kd protein (SCP-2) and a larger 58 Kd protein (SCP-x). The former is found in the cytoplasm or the mitochondria and is involved in lipid transport; the latter is found in peroxisomes. The C-terminal part of SCP-x is identical to SCP-2 while the N-terminal portion is evolutionary related to thiolases.
Mechanism
Thioesters are more reactive than oxygen esters and are common intermediates in fatty-acid metabolism. These thioesters are made by conjugating the fatty acid with the free SH group of the pantetheinePantetheine
Pantetheine is the cysteamine amide analogue of pantothenic acid . The dimer of this compound, pantethine is more commonly known, and is considered to be a more potent form of vitamin B5 than pantothenic acid. Pantetheine is an intermediate in the production of coenzyme A by the body....
moiety of either coenzyme A
Coenzyme A
Coenzyme A is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All sequenced genomes encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it as a substrate...
(CoA) or acyl carrier protein
Acyl carrier protein
The acyl carrier protein is an important component in both fatty acid and polyketide biosynthesis with the growing chain bound during synthesis as a thiol ester at the distal thiol of a 4'-phosphopantethiene moiety...
(ACP).
All thiolases, whether they are biosynthetic or degradative in vivo, preferentially catalyze the degradation of 3-ketoacyl-CoA to form acetyl-CoA and a shortened acyl-CoA species, but are also capable of catalyzing the reverse Claisen condensation
Claisen condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone...
reaction. It is well established from studies on the biosynthetic thiolase from Z. ramigera that the thiolase reaction occurs in two steps and follows ping-pong kinetics. In the first step of both the degradative and biosynthetic reactions, the nucleophilic Cys89 (or its equivalent) attacks the acyl-CoA (or 3-ketoacyl-CoA) substrate,leading to the formation of a covalent acyl-CoA intermediate. In the second step, the addition of CoA (in the degradative reaction) or acetyl-CoA (in the biosynthetic reaction) to the acyl–enzyme intermediate triggers the release of the product from the enzyme.
Structure
Most enzyme of the thiolase super family are dimersProtein dimer
In biochemistry, a dimer is a macromolecular complex formed by two, usually non-covalently bound, macromolecules like proteins or nucleic acids...
. However, monomers have not been observed. Tetrameters are observed only in the thiolase subfamily and, in these cases, the dimers have dimerized to become tetramers. The crystal structure of the tetrameric biosynthetic thiolase from Zoogloea ramigera has been determined at 2.0 Å resolution. The structure contains a striking and novel ‘cage-like’ tetramerization motif, which allows for some hinge motion of the two tight dimers with respect to each other. The enzyme tetramer is acetylated at Cys89 and has a CoA molecule bound in each of its
active-site pockets.
Biological function
In eukaryotic cells, especially in mammalian cells, thiolases exhibit diversity in intracellular localization related to their metabolic functions as well as in substrate specificity. For example, they contribute to fatty-acid β-oxidation in peroxisomes and mitochondria, ketone body metabolism in mitochondria, and the early steps of mevalonate pathway in peroxisomes and cytoplasmCytoplasm
The cytoplasm is a small gel-like substance residing between the cell membrane holding all the cell's internal sub-structures , except for the nucleus. All the contents of the cells of prokaryote organisms are contained within the cytoplasm...
. In addition to biochemical investigations, analyses of genetic disorders have made clear the basis of their functions. Genetic studies have also started to disclose the physiological functions of thiolases in the yeast Saccharomyces cerevisiae. Thiolase is of central importance in key enzymatic pathways such as fatty-acid, steroid and polyketide synthesis. The detailed understanding of its structural biology is of great medical relevance, for example, for a better understanding of the diseases caused by genetic deficiencies of these enzymes and for the development of new antibiotics. Harnessing the complicated catalytic versatility of the polyketide synthases for the synthesis of biologically and medically relevant natural products is also an important future perspective of the studies of the enzymes of this superfamily.
Disease relevance
Mitochondrial acetoacetyl-CoA thiolase deficiency, known earlier as β-ketothiolase deficiencyBeta-ketothiolase deficiency
Beta-ketothiolase deficiency is a rare, autosomal recessive metabolic disorder in which the body cannot properly process the amino acid isoleucine or the products of lipid breakdown....
, is an inborn error of metabolism involving isoleucine catabolism and ketone body metabolism. The major clinical manifestations of this disorder are intermittent ketoacidosis
Ketoacidosis
Ketoacidosis is a metabolic state associated with high concentrations of ketone bodies, formed by the breakdown of fatty acids and the deamination of amino acids. The two common ketones produced in humans are acetoacetic acid and β-hydroxybutyrate....
but the long-term clinical consequences, apparently benign, are not well documented. Mitochondrial acetoacetyl-CoA thiolase deficiency deficiency is easily diagnosed by urinary organic acid analysis and can be confirmed by enzymatic analysis of cultured skin fibroblasts or blood leukocytes.
β-Ketothiolase Deficiency has a variable presentation. Most affected patients present between 5 and 24 months of age with symptoms of severe ketoacidosis. Symptoms can be initiated by a dietary protein load, infection or fever. Symptoms progress from vomiting to dehydration and ketoacidosis. Neutropenia and thrombocytopenia may be present, as can moderate hyperammonemia. Blood glucose is typically normal, but can be low or high in acute episodes. Developmental delay may occur, even before the first acute episode, and bilateral striatal necrosis
Necrosis
Necrosis is the premature death of cells in living tissue. Necrosis is caused by factors external to the cell or tissue, such as infection, toxins, or trauma. This is in contrast to apoptosis, which is a naturally occurring cause of cellular death...
of the basal ganglia
Basal ganglia
The basal ganglia are a group of nuclei of varied origin in the brains of vertebrates that act as a cohesive functional unit. They are situated at the base of the forebrain and are strongly connected with the cerebral cortex, thalamus and other brain areas...
has been seen on brain MRI.

