
Thermodynamic reaction control
Encyclopedia
Thermodynamic reaction control or kinetic reaction control in a chemical reaction
can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity
. The distinction is relevant when product A forms faster (which is called the kinetically controlled product) than product B because the activation energy
for product A is lower than that for product B, yet product B is more stable (this is called the thermodynamically controlled product)
The conditions of the reaction, such as temperature, pressure, or solvent, affect which reaction pathway may be favored: either the kinetically controlled or the thermodynamically controlled one. Note this is only true if the activation energy of the two pathways differ, with one pathway having a lower Ea (energy of activation) than the other.
Prevalence of thermodynamic or kinetic control determines the final composition of the product when these competing reaction pathways lead to different products. The reaction conditions as mentioned above influence the selectivity
of the reaction - i.e., which pathway is taken.
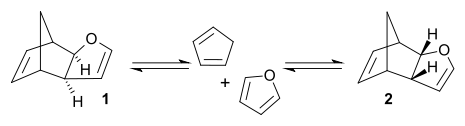
of cyclopentadiene
with furan
can produce two isomeric products. At room temperature
, kinetic reaction control prevails and the less stable endo isomer 2 is the main reaction product. At 81°C and after long reaction times, the chemical equilibrium
can assert itself and the thermodynamically more stable exo isomer 1 is formed. The exo product is more stable by virtue of a lower degree of steric congestion
, while the endo product is favoured by orbital overlap in the transition state
.
of an enolate ion, the kinetic product is the enol
and the thermodynamic product is a ketone
or aldehyde
. Carbonyl compounds and their enols interchange rapidly by proton
transfers catalyzed by acid
s or base
s, even in trace amounts, in this case mediated by the enolate or the proton source.
In the deprotonation
of an unsymmetrical ketone
, the kinetic product is the enolate resulting from removal of the most accessible α-H while the thermodynamic product has the more highly substituted enolate moiety. Use of low temperatures and sterically demanding base
s increases the kinetic selectivity. Here, the difference in pKb
between the base and the enolate is so large that the reaction is essentially irreversible, so the equilibration leading to the thermodynamic product is likely a proton exchange occurring during the addition between the kinetic enolate and as-yet-unreacted ketone. An inverse addition (adding ketone to the base) with rapid mixing would minimize this. The position of the equilibrium will depend on the countercation and solvent.
reaction of hydrogen bromide
to 1,3-butadiene
above room temperature leads predominantly to the thermodynamically more stable 1,4 adduct, 1-bromo-2-butene, but decreasing the reaction temperature to below room temperature favours the kinetic 1,2 adduct, 3-bromo-1-butene.
 (equation 1)
(equation 1)
 (equation 2)
(equation 2)
and a fulvene
first reported in 1929 by Otto Diels
and Kurt Alder
. They observed that while the endo isomer is formed more rapidly, longer reaction times, as well as relatively elevated temperatures, result in higher exo / endo ratios which had to be considered in the light of the remarkable stability of the exo-compound on the one hand and the very facile dissociation of the endo isomer on the other.
C. K. Ingold with E. D. Hughes and G. Catchpole independently described a thermodynamic and kinetic reaction control model in 1948. They were reinvestigating a certain allylic rearrangement
reported in 1930 by Jakob Meisenheimer
. Solvolysis of gamma-phenylallyl chloride with AcOK
in acetic acid was found to give a mixture of the gamma and the alpha acetate with the latter converting to the first by equilibration. This was interpreted as a case in the field of anionotropy of the phenomenon, familiar in prototropy, of the distinction between kinetic and thermodynamic control in ion-recombination.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity
Selectivity
Selectivity may refer to:* Selectivity , in radio transmission* Binding selectivity, in pharmacology* Functional selectivity, in pharmacology* Socioemotional selectivity theory, in social psychology...
. The distinction is relevant when product A forms faster (which is called the kinetically controlled product) than product B because the activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
for product A is lower than that for product B, yet product B is more stable (this is called the thermodynamically controlled product)
The conditions of the reaction, such as temperature, pressure, or solvent, affect which reaction pathway may be favored: either the kinetically controlled or the thermodynamically controlled one. Note this is only true if the activation energy of the two pathways differ, with one pathway having a lower Ea (energy of activation) than the other.
Prevalence of thermodynamic or kinetic control determines the final composition of the product when these competing reaction pathways lead to different products. The reaction conditions as mentioned above influence the selectivity
Selectivity
Selectivity may refer to:* Selectivity , in radio transmission* Binding selectivity, in pharmacology* Functional selectivity, in pharmacology* Socioemotional selectivity theory, in social psychology...
of the reaction - i.e., which pathway is taken.
In Diels-Alder reactions
The Diels-Alder reactionDiels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
of cyclopentadiene
Cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction...
with furan
Furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. The class of compounds containing such rings are also referred to as furans....
can produce two isomeric products. At room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
, kinetic reaction control prevails and the less stable endo isomer 2 is the main reaction product. At 81°C and after long reaction times, the chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
can assert itself and the thermodynamically more stable exo isomer 1 is formed. The exo product is more stable by virtue of a lower degree of steric congestion
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
, while the endo product is favoured by orbital overlap in the transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
.

In enolate chemistry
In the protonationProtonation
In chemistry, protonation is the addition of a proton to an atom, molecule, or ion. Some classic examples include*the protonation of water by sulfuric acid:*the protonation of isobutene in the formation of a carbocation:2C=CH2 + HBF4 → 3C+ + BF4−*the protonation of ammonia in the...
of an enolate ion, the kinetic product is the enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
and the thermodynamic product is a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
or aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
. Carbonyl compounds and their enols interchange rapidly by proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
transfers catalyzed by acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
s or base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
s, even in trace amounts, in this case mediated by the enolate or the proton source.
In the deprotonation
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
of an unsymmetrical ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
, the kinetic product is the enolate resulting from removal of the most accessible α-H while the thermodynamic product has the more highly substituted enolate moiety. Use of low temperatures and sterically demanding base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
s increases the kinetic selectivity. Here, the difference in pKb
PKB
PKB is a three-letter abbreviation that may refer to:* Państwowy Korpus Bezpieczeństwa, a Polish underground police force during World War II* The National Awakening Party of Indonesia, from the abbreviation for its name in Indonesian...
between the base and the enolate is so large that the reaction is essentially irreversible, so the equilibration leading to the thermodynamic product is likely a proton exchange occurring during the addition between the kinetic enolate and as-yet-unreacted ketone. An inverse addition (adding ketone to the base) with rapid mixing would minimize this. The position of the equilibrium will depend on the countercation and solvent.
- If a much weaker base is used, the deprotonation will be incomplete, and there will be an equilibrium between reactants and products. Thermodynamic control is obtained, however the reaction remains incomplete unless the product enolate is trapped, as in the example below. Since H transfers are very fast, the trapping reaction being slower, the ratio of trapped products largely mirrors the deprotonation equilibrium.
In electrophilic additions
The electrophilic additionElectrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where, in a chemical compound, a π bond is broken and two new σ bonds are formed...
reaction of hydrogen bromide
Hydrogen bromide
Hydrogen bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation. Hydrogen bromide and...
to 1,3-butadiene
1,3-Butadiene
1,3-Butadiene is a simple conjugated diene with the formula C4H6. It is an important industrial chemical used as a monomer in the production of synthetic rubber. When the word butadiene is used, most of the time it refers to 1,3-butadiene....
above room temperature leads predominantly to the thermodynamically more stable 1,4 adduct, 1-bromo-2-butene, but decreasing the reaction temperature to below room temperature favours the kinetic 1,2 adduct, 3-bromo-1-butene.
- The rationale for the differing selectivities is as follows: Both products result from MarkovnikovMarkovnikov's ruleIn organic chemistry, Markovnikov's rule or Markownikoff's rule is an observation based on Zaitsev's rule. It was formulated by the Russian chemist Vladimir Vasilevich Markovnikov in 1870....
protonation at position 1, resulting in a resonanceResonance (chemistry)In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
-stabilized allylic cation. The 1,4 adduct places the larger Br atom at a less congested site and includes a more highly substituted alkene moiety, while the 1,2 adduct is the result of the attack by the nucleophile (Br-) at the carbonCarbonCarbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
of the allylic cation bearing the greatest positive charge (the more highly substituted carbon).
Characteristics
- In every reaction, the first product formed is that which is most easily formed. Thus, every reaction a priori starts under kinetic control.
- A necessary condition for thermodynamic control is reversibility or a mechanism permitting the equilibration between products. Reactions are considered to take place under thermodynamic reaction control when the reverse reaction is sufficiently rapid that the equilibriumChemical equilibriumIn a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
establishes itself within the alloted reaction time. In this way, the thermodynamically more stable product is always favoured.
- Under kinetic reaction control, the forward reaction is faster than the reverse reaction. After reaction time t, the product ratio is the ratio of rate constants k and thus a function of the difference in activation energies Ea or ΔG‡:
 (equation 1)
(equation 1)- Unless equilibration is prevented, pure kinetic control is practically impossible, because equilibration will have started before the reactants will have been entirely consumed.
- Under pure thermodynamic reaction control, when the equilibrium has been reached, the product distribution will be a function of the stabilities G°. After an infinite amount of reaction time, the ratio of product concentrations will equal the equilibrium constant Keq and therefore be a function of the difference in Gibbs free energiesGibbs free energyIn thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
,
 (equation 2)
(equation 2)- In general, short reaction times favour kinetic control, whereas longer reaction times favour thermodynamic reaction control. Low temperatures will enhance the selectivity under both sets of conditions, since T is in the denominator in both cases. The ideal temperature to optimise the yield of the fastest-forming product will be the lowest temperature that will ensure reaction completion in a reasonable amount of time. The ideal temperature for a reaction under thermodynamic control is the lowest temperature at which equilibrium will be reached in a reasonable amount of time. If needed, the selectivity can be increased by then slowly cooling the reaction mixture to shift the equilibrium further toward the most stable product. When the difference in product stability is very large, the thermodynamically controlled product can dominate even under relatively vigorous reaction conditions.
- If a reaction is under thermodynamic control at a given temperature, it will also be under thermodynamic control at a higher temperature for the same reaction time.
- In the same manner, if a reaction is under kinetic control at a given temperature, it will also be under kinetic control at any lower temperature for the same reaction time.
- If one presumes that a new reaction will be a priori under kinetic control, one can detect the presence of an equilibration mechanism (and therefore the possibility of thermodynamic control) if the product distribution:
- changes over time,
- shows one product to be dominant at one temperature while another dominates at a different temperature (inversion of dominance), or
- changes with temperature but is not consistent with equation 1, that is a change in temperature (without changing the reaction time) causes a change in the product ratio
 that is larger or smaller than would be expected from the change in temperature alone, assuming that
that is larger or smaller than would be expected from the change in temperature alone, assuming that  is largely invariant with temperature over a modest temperature range.
is largely invariant with temperature over a modest temperature range.
- In the same way, one can detect the possibility of kinetic control if a temperature change causes a change in the product ratio that is inconsistent with equation 2, assuming that
 is largely invariant with temperature over a modest temperature range.
is largely invariant with temperature over a modest temperature range.
History
The first to report on the relationship between kinetic and thermodynamic stability were R.B. Woodward and Harold Baer in 1944. They were re-investigating a reaction between maleic anhydrideMaleic anhydride
Maleic anhydride is an organic compound with the formula C2H22O. It is the acid anhydride of maleic acid and in its pure state it is a colourless or white solid with an acrid odour....
and a fulvene
Fulvene
Fulvene is one of several hydrocarbons with the same formula as benzene, C6H6. Fulvenes include the derivatives of this simple hydrocarbon, which itself is rarely encountered. Thiele is credited with discovering the scope of the reaction between cyclopentadiene and aldehydes and ketones that...
first reported in 1929 by Otto Diels
Otto Diels
Otto Paul Hermann Diels was a German chemist. He was the son of a professor of philology at the University of Berlin, where he himself earned his doctorate in chemistry, in the group of Emil Fischer....
and Kurt Alder
Kurt Alder
Kurt Alder was a German chemist and Nobel laureate.-Biography:Alder was born in the industrial area of Königshütte, Silesia , where he received his early schooling...
. They observed that while the endo isomer is formed more rapidly, longer reaction times, as well as relatively elevated temperatures, result in higher exo / endo ratios which had to be considered in the light of the remarkable stability of the exo-compound on the one hand and the very facile dissociation of the endo isomer on the other.
C. K. Ingold with E. D. Hughes and G. Catchpole independently described a thermodynamic and kinetic reaction control model in 1948. They were reinvestigating a certain allylic rearrangement
Allylic rearrangement
An allylic rearrangement or allylic shift is an organic reaction in which the double bond in an allyl chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution....
reported in 1930 by Jakob Meisenheimer
Jakob Meisenheimer
Jakob Meisenheimer was a German chemist. He made numerous contributions to organic chemistry, the most famous being his proposed structure for a group of compounds now named Meisenheimer complex. He also proposed the mechanism of the Beckmann rearrangement. Later in his career, he reported the...
. Solvolysis of gamma-phenylallyl chloride with AcOK
Potassium acetate
Potassium acetate is the potassium salt of acetic acid.-Preparation:It can be prepared by reacting a potassium-containing base such as potassium hydroxide or potassium carbonate with acetic acid:...
in acetic acid was found to give a mixture of the gamma and the alpha acetate with the latter converting to the first by equilibration. This was interpreted as a case in the field of anionotropy of the phenomenon, familiar in prototropy, of the distinction between kinetic and thermodynamic control in ion-recombination.

