Stevens rearrangement
Encyclopedia
The Stevens rearrangement in organic chemistry
is an organic reaction
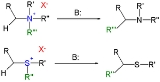
converting quaternary ammonium salts and sulfonium salts to the corresponding amine
s or sulfide
s in presence of a strong base
in a 1,2-rearrangement
.
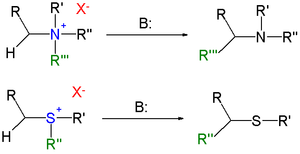 The reactants can be obtained by alkylation
The reactants can be obtained by alkylation
of the corresponding amines and sulfides. The substituent
R next the amine methylene
group is an electron-withdrawing group.
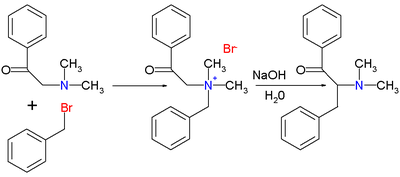
The original 1928 publication by T.S. Stevens concerned the reaction of 1-phenyl-2- (N, N-dimethyl) ethanone with benzyl bromide
to the ammonium salt followed by the rearrangement reaction with sodium hydroxide in water to the rearranged amine.
 A 1932 publication described the corresponding sulfur reaction.
A 1932 publication described the corresponding sulfur reaction.
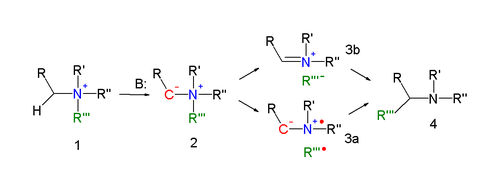
for the Stevens rearrangement (explained for the nitrogen reaction) is the formation of an ylide
after deprotonation
of the ammonium salt by a strong base. Deprotonation is aided by electron-withdrawing properties of substituent R. Several reaction modes exist for the actual rearrangement reaction.
A concerted reaction
requires an antarafacial reaction mode but since the migrating group displays retention of configuration this mechanism is unlikely.
In an alternative reaction mechanism the N-C bond of the leaving group is homolytically
cleaved to form a di-radical pair (3a). In order to explain the observed retention of configuration, the presence of a solvent cage is invoked. Another possibility is the formation of a cation-anion pair (3b), also in a solvent cage.
and the Hofmann elimination
.
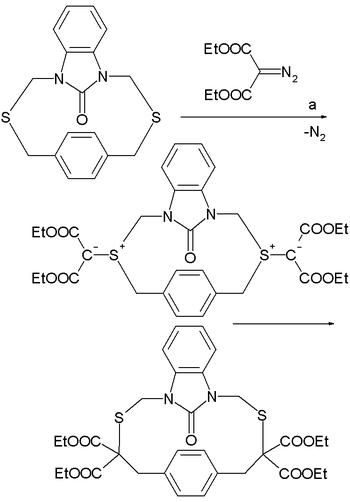
In one application a double-Stevens rearrangement expands a cyclophane
ring . The ylide is prepared in situ
by reaction of the diazo
compound ethyl diazomalonate with a sulfide
catalyzed by dirhodium tetraacetate in reflux
ing xylene
.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is an organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
converting quaternary ammonium salts and sulfonium salts to the corresponding amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s or sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
s in presence of a strong base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
in a 1,2-rearrangement
1,2-rearrangement
A 1,2-rearrangement or 1,2-migration or 1,2-shift or Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atoms but moves over larger distances are possible...
.

Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
of the corresponding amines and sulfides. The substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
R next the amine methylene
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
group is an electron-withdrawing group.
The original 1928 publication by T.S. Stevens concerned the reaction of 1-phenyl-2- (N, N-dimethyl) ethanone with benzyl bromide
Benzyl bromide
Benzyl bromide, or α-bromotoluene, is an organic compound consisting of a benzene ring substituted with a bromomethyl group. It can be prepared by the bromination of toluene at room temperature in air, using manganese oxide as a heterogeneous catalyst...
to the ammonium salt followed by the rearrangement reaction with sodium hydroxide in water to the rearranged amine.

Reaction mechanism
Key in the reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
for the Stevens rearrangement (explained for the nitrogen reaction) is the formation of an ylide
Ylide
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom directly attached to a hetero atom with a formal positive charge , and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compounds...
after deprotonation
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
of the ammonium salt by a strong base. Deprotonation is aided by electron-withdrawing properties of substituent R. Several reaction modes exist for the actual rearrangement reaction.
A concerted reaction
Concerted reaction
In chemistry, a concerted reaction is a chemical reaction in which all bond breaking and bond making occurs in a single step. Reactive intermediates or other unstable high energy intermediates are not involved. Concerted reaction rates tend not to depend on solvent polarity ruling out large buildup...
requires an antarafacial reaction mode but since the migrating group displays retention of configuration this mechanism is unlikely.
In an alternative reaction mechanism the N-C bond of the leaving group is homolytically
Homolysis
In general it means breakdown to equal pieces There are separate meanings for the word in chemistry and biology.-Homolysis in chemistry:...
cleaved to form a di-radical pair (3a). In order to explain the observed retention of configuration, the presence of a solvent cage is invoked. Another possibility is the formation of a cation-anion pair (3b), also in a solvent cage.

Scope
Competing reactions are the Sommelet-Hauser rearrangementSommelet-Hauser rearrangement
The Sommelet–Hauser rearrangement is a rearrangement reaction of certain benzyl quaternary ammonium salts...
and the Hofmann elimination
Hofmann elimination
Hofmann elimination is a process where an amine is reacted to create a tertiary amine and an alkene by treatment with excess methyl iodide followed by treatment with silver oxide, water, and heat.After the first step, a quaternary ammonium iodide salt is created...
.
In one application a double-Stevens rearrangement expands a cyclophane
Cyclophane
A cyclophane is a hydrocarbon consisting of an aromatic unit and an aliphatic chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known...
ring . The ylide is prepared in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
by reaction of the diazo
Diazo
Diazo refers to a type of organic compound called diazo compound that has two linked nitrogen atoms as a terminal functional group. The general formula is R2C=N2. The simplest example of a diazo compound is diazomethane...
compound ethyl diazomalonate with a sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
catalyzed by dirhodium tetraacetate in reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
ing xylene
Xylene
Xylene encompasses three isomers of dimethylbenzene. The isomers are distinguished by the designations ortho- , meta- , and para- , which specify to which carbon atoms the two methyl groups are attached...
.