_chloride.gif)
Mercury(I) chloride
Encyclopedia
Mercury chloride is the chemical compound
with the formula Hg2Cl2. Also known as calomel (a mineral form, rarely found in nature) or mercurous chloride, this dense white or yellowish-white, odorless solid is the principal example of a mercury
(I) compound. It is a component of reference electrode
s in electrochemistry
.
καλός beautiful, and μέλας black. This name (somewhat surprising for a white compound) is probably due to its characteristic disproportionation
reaction with ammonia
, which gives a spectacular black coloration due to the finely dispersed metallic mercury
formed. It is also referred to as the mineral horn quicksilver or horn mercury. Calomel was taken internally and used as a laxative and disinfectant, as well as in the treatment of syphilis, until the early 20th century.
Mercury became a popular remedy for a variety of physical and mental ailments during the age of "heroic medicine
." It was used by doctors in America throughout the 18th century, and during the revolution, to make patients regurgitate and release their body from "impurities". Benjamin Rush
, a famed physician in colonial Philadelphia and signatory to the Declaration of Independence
, was one particular well-known advocate of mercury in medicine and famously used calomel to treat sufferers of yellow fever
during its outbreak in the city in 1793. Calomel was given to patients as a purgative until they began to salivate. However, it was often administered to patients in such great quantities that their hair and teeth fell out. Shortly after yellow fever struck Philadelphia, the disease broke out in Jamaica. A war of words broke out in the newspapers concerning the best treatment for yellow fever; bleeding or calomel. Anecdotal evidence indicates calomel was more effective than bleeding.
is shown below:
The Hg–Hg bond length of 253 pm (Hg–Hg in the metal is 300 pm) and the Hg–Cl bond length in the linear Hg2Cl2 unit is 243 pm. The overall coordination of each Hg atom is octahedral as, in addition to the two nearest neighbours, there are four other Cl atoms at 321 pm. Longer mercury polycations
exist.
It can be prepared via metathesis
reaction involving aqueous mercury(I) nitrate
using various chloride sources including NaCl or HCl.
Ammonia
causes Hg2Cl2 to disproportionate
:
, taking advantage of the ease of its oxidation and reduction reactions. The calomel electrode is a reference electrode
, especially in older publications. Over the past 50 years, it has been superseded by the silver/silver chloride (Ag/AgCl) electrode. Although the mercury electrodes have been widely abandoned due to the dangerous nature of mercury
, many chemists believe they are still more accurate and are not dangerous as long as they are handled properly. The differences in experimental potentials vary little from literature values. Other electrodes can vary by 70 to 100 millivolts.
and elemental mercury upon exposure to UV light.
The formation of Hg can be used to calculate the number of photons in the light beam, by the technique of actinometry
. By utilizing a light reaction in the presence of mercury(II) chloride
and ammonium oxalate
, mercury(I) chloride, ammonium chloride
and carbon dioxide
is produced.
This particular reaction was discovered by J.M. Eder (hence the name Eder reaction) in 1880 and reinvestigated by W. E. Rosevaere in 1929
, Hg2Br2, is a light yellow, whereas mercury(I) iodide
, Hg2I2, is greenish in colour. Both are poorly soluble. Mercury(I) fluoride
is unstable in the absence of a strong acid.
, although due to its low solubility in water it is generally less dangerous than its mercuric chloride counterpart. It was used in medicine as a diuretic
and purgative (laxative) in the United States
from the early 1830s through the 1860s. Calomel was also a common ingredient in teething
powders in Britain up until 1954, causing widespread mercury poisoning in the form of pink disease, which at the time had a mortality rate of 1 in 10. These medicinal uses were later discontinued when the compound's toxicity was discovered.
It has also found uses in cosmetics as soaps and skin lightening creams, but these preparations are now illegal to manufacture or import in many countries including the U.S., Canada, Japan and the European Union. A study of workers involved in the production of these preparations showed that the sodium salt of 2,3-dimercapto-1-propanesulfonic acid
(DMPS) was effective in lowering the body burden of mercury and in decreasing the urinary mercury concentration to normal levels.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with the formula Hg2Cl2. Also known as calomel (a mineral form, rarely found in nature) or mercurous chloride, this dense white or yellowish-white, odorless solid is the principal example of a mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
(I) compound. It is a component of reference electrode
Reference electrode
A reference electrode is an electrode which has a stable and well-known electrode potential. The high stability of the electrode potential is usually reached by employing a redox system with constant concentrations of each participants of the redox reaction.There are many ways reference...
s in electrochemistry
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
.
History
The name calomel is thought to come from the GreekGreek language
Greek is an independent branch of the Indo-European family of languages. Native to the southern Balkans, it has the longest documented history of any Indo-European language, spanning 34 centuries of written records. Its writing system has been the Greek alphabet for the majority of its history;...
καλός beautiful, and μέλας black. This name (somewhat surprising for a white compound) is probably due to its characteristic disproportionation
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
reaction with ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, which gives a spectacular black coloration due to the finely dispersed metallic mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
formed. It is also referred to as the mineral horn quicksilver or horn mercury. Calomel was taken internally and used as a laxative and disinfectant, as well as in the treatment of syphilis, until the early 20th century.
Mercury became a popular remedy for a variety of physical and mental ailments during the age of "heroic medicine
Heroic medicine
Heroic medicine is a twentieth century term for aggressive medical practices or methods of treatment used until the mid-nineteenth century, and usually refers to treatments that scientific advances later replaced....
." It was used by doctors in America throughout the 18th century, and during the revolution, to make patients regurgitate and release their body from "impurities". Benjamin Rush
Benjamin Rush
Benjamin Rush was a Founding Father of the United States. Rush lived in the state of Pennsylvania and was a physician, writer, educator, humanitarian and a Christian Universalist, as well as the founder of Dickinson College in Carlisle, Pennsylvania....
, a famed physician in colonial Philadelphia and signatory to the Declaration of Independence
Declaration of independence
A declaration of independence is an assertion of the independence of an aspiring state or states. Such places are usually declared from part or all of the territory of another nation or failed nation, or are breakaway territories from within the larger state...
, was one particular well-known advocate of mercury in medicine and famously used calomel to treat sufferers of yellow fever
Yellow fever
Yellow fever is an acute viral hemorrhagic disease. The virus is a 40 to 50 nm enveloped RNA virus with positive sense of the Flaviviridae family....
during its outbreak in the city in 1793. Calomel was given to patients as a purgative until they began to salivate. However, it was often administered to patients in such great quantities that their hair and teeth fell out. Shortly after yellow fever struck Philadelphia, the disease broke out in Jamaica. A war of words broke out in the newspapers concerning the best treatment for yellow fever; bleeding or calomel. Anecdotal evidence indicates calomel was more effective than bleeding.
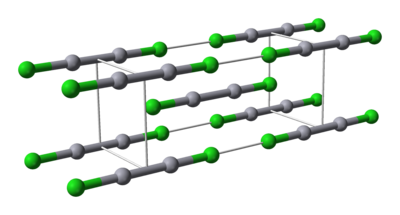
Properties
Mercury is unique among the group 12 metals for its ability to form the M–M bond so readily. Hg2Cl2 is a linear molecule. The unit cell of the crystal structureCrystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
is shown below:
 |
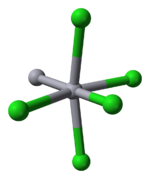 |
The Hg–Hg bond length of 253 pm (Hg–Hg in the metal is 300 pm) and the Hg–Cl bond length in the linear Hg2Cl2 unit is 243 pm. The overall coordination of each Hg atom is octahedral as, in addition to the two nearest neighbours, there are four other Cl atoms at 321 pm. Longer mercury polycations
Mercury polycations
Mercury polycations are polyatomic cations that contain only mercury atoms. The best known example is the ion, found in mercury compounds...
exist.
Preparation and reactions
Mercurous chloride forms by the reaction of elemental mercury and mercuric chloride:-
- Hg + HgCl2 → Hg2Cl2
It can be prepared via metathesis
Metathesis (chemistry)
Salt metathesis is a molecular process involving the exchange of bonds between the two reacting chemical species, which results in the creation of products with similar or identical bonding affiliations. This is represented by the general reaction scheme:These chemical species can either be ionic...
reaction involving aqueous mercury(I) nitrate
Mercury(I) nitrate
Mercury nitrate is a chemical compound with the formula Hg22. It is used in the preparation of other mercury compounds, and, like all other mercury compounds, it is toxic.-Reactions:...
using various chloride sources including NaCl or HCl.
-
- 2HCl + Hg2(NO3)2 → Hg2Cl2 + 2HNO3
Ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
causes Hg2Cl2 to disproportionate
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
:
-
- Hg2Cl2 + 2NH3 → Hg + Hg(NH2)Cl + NH4Cl
Calomel electrode
Mercurous chloride is employed extensively in electrochemistryElectrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
, taking advantage of the ease of its oxidation and reduction reactions. The calomel electrode is a reference electrode
Reference electrode
A reference electrode is an electrode which has a stable and well-known electrode potential. The high stability of the electrode potential is usually reached by employing a redox system with constant concentrations of each participants of the redox reaction.There are many ways reference...
, especially in older publications. Over the past 50 years, it has been superseded by the silver/silver chloride (Ag/AgCl) electrode. Although the mercury electrodes have been widely abandoned due to the dangerous nature of mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
, many chemists believe they are still more accurate and are not dangerous as long as they are handled properly. The differences in experimental potentials vary little from literature values. Other electrodes can vary by 70 to 100 millivolts.
Photochemistry
Mercurous chloride decomposes into mercury(II) chlorideMercury(II) chloride
Mercury chloride or mercuric chloride , is the chemical compound with the formula HgCl2. This white crystalline solid is a laboratory reagent and a molecular compound. It is no longer used for medicinal purposes Mercury(II) chloride or mercuric chloride (formerly corrosive sublimate), is the...
and elemental mercury upon exposure to UV light.
-
- Hg2Cl2 → HgCl2 + Hg
The formation of Hg can be used to calculate the number of photons in the light beam, by the technique of actinometry
Actinometer
Actinometers are instruments used to measure the heating power of radiation. They are used in meteorology to measure solar radiation as pyrheliometers.An actinometer is a chemical system or physical device which determines the number of...
. By utilizing a light reaction in the presence of mercury(II) chloride
Mercury(II) chloride
Mercury chloride or mercuric chloride , is the chemical compound with the formula HgCl2. This white crystalline solid is a laboratory reagent and a molecular compound. It is no longer used for medicinal purposes Mercury(II) chloride or mercuric chloride (formerly corrosive sublimate), is the...
and ammonium oxalate
Ammonium oxalate
Ammonium oxalate, C2H8N2O4, is an oxalate salt with ammonia. It is a constituent of some types of kidney stone. Found also in guano....
, mercury(I) chloride, ammonium chloride
Ammonium chloride
Ammonium chloride NH4Cl is an inorganic compound with the formula NH4Cl. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride...
and carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
is produced.
-
- 2HgCl2 + (NH4)2C2O4 + Light → Hg2Cl2(s) + 2[NH4+][Cl−] + 2CO2
This particular reaction was discovered by J.M. Eder (hence the name Eder reaction) in 1880 and reinvestigated by W. E. Rosevaere in 1929
Related mercury(I) compounds
Mercury(I) bromideMercury(I) bromide
Mercury bromide or mercurous bromide is the chemical compound composed of mercury and bromine with the formula Hg2Br2. It changes color from white to yellow when heated and fluoresces orange when exposed to ultraviolet light...
, Hg2Br2, is a light yellow, whereas mercury(I) iodide
Mercury(I) iodide
Mercury iodide is a chemical compound of mercury and iodine. The chemical formula is Hg2I2. It is photosensitive and decomposes easily to mercury and HgI2.-Synthesis:Mercury iodide can be prepared by direct combination of mercury and iodine:...
, Hg2I2, is greenish in colour. Both are poorly soluble. Mercury(I) fluoride
Mercury(I) fluoride
Mercury fluoride or mercurous fluoride is the chemical compound composed of mercury and fluorine with the formula Hg2F2. It consists of small yellow cubic crystals which turn black when exposed to light.-Reactions:...
is unstable in the absence of a strong acid.
Safety considerations
Mercurous chloride is toxicMercury poisoning
Mercury poisoning is a disease caused by exposure to mercury or its compounds. Mercury is a heavy metal occurring in several forms, all of which can produce toxic effects in high enough doses...
, although due to its low solubility in water it is generally less dangerous than its mercuric chloride counterpart. It was used in medicine as a diuretic
Diuretic
A diuretic provides a means of forced diuresis which elevates the rate of urination. There are several categories of diuretics. All diuretics increase the excretion of water from bodies, although each class does so in a distinct way.- Medical uses :...
and purgative (laxative) in the United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
from the early 1830s through the 1860s. Calomel was also a common ingredient in teething
Teething
Teething is the process by which an infant's first teeth sequentially appear by emerging through the gums. Teething may start as early as three months or as late, in some cases, as twelve months. The typical time frame for the first teeth to appear is somewhere between six and nine months...
powders in Britain up until 1954, causing widespread mercury poisoning in the form of pink disease, which at the time had a mortality rate of 1 in 10. These medicinal uses were later discontinued when the compound's toxicity was discovered.
It has also found uses in cosmetics as soaps and skin lightening creams, but these preparations are now illegal to manufacture or import in many countries including the U.S., Canada, Japan and the European Union. A study of workers involved in the production of these preparations showed that the sodium salt of 2,3-dimercapto-1-propanesulfonic acid
2,3-Dimercapto-1-propanesulfonic acid
2,3-Dimercapto-1-propanesulfonic acid and its sodium salt are chelating agents that form complexes with various heavy metals. They are related to dimercaprol, which is another chelating agent....
(DMPS) was effective in lowering the body burden of mercury and in decreasing the urinary mercury concentration to normal levels.

