
Ritter reaction
Encyclopedia
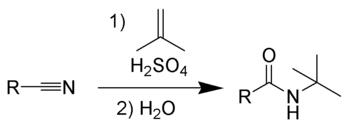
The Ritter reaction is a chemical reaction
that transforms a nitrile
into a N-alkyl amide
using various alkylating reagents, for example, strong acid and isobutylene
.
 Primary, secondary, tertiary, and benzylic alcohol
Primary, secondary, tertiary, and benzylic alcohol
s, as well as tert-butyl acetate, also successfully react with nitriles in the presence of strong acids to form amides via the Ritter reaction.
from Columbia University
. In 1948, P. Paul Minieri
, Ritter's student, submitted work on the reaction as his Ph.D. thesis. Ritter and Minieri collaborated together to perform the experiment in the Microchemistry Laboratory at NYU. Ritter and Minieri described the reaction of a nitrile
being transformed, in the presence of sulfuric acid
and alkenes, into an amide
. They characterized the product to confirm that it was a N-alkyl amide through the Kjeldahl method
, which quantitatively determines nitrogen
presence in chemical substances. Although it was developed 62 years ago, the reaction still has significance today due to its applicability and reproducibility of amides via stabilized carbocations.
2 or covalent species to the nitrile
. The resulting nitrilium ion 3 is hydrolyzed
by water to the desired amide 5.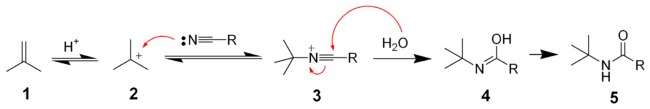
’s industrial-scale synthesis of anti-HIV
drug Crixivan (indinavir); the production of the falcipain-2 inhibitor PK 11195; the synthesis of the alkaloid
aristotelone; and synthesis of Amantadine
, an antiviral and antiparkinsonian drug. Other applications of the Ritter reaction include synthesis of dopamine receptor
ligands and production of amphetamine
from allylbenzene.
A problem with the Ritter reaction is the necessity of an extremely strong acid catalyst in order to produce the carbocation
. This poses an environmental risk, as the acids are extremely corrosive and also cannot be reused. However, other methods have been proposed in order promote carbocation
formation, including photosensitized electron transfer
or direct photolysis.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
that transforms a nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
into a N-alkyl amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
using various alkylating reagents, for example, strong acid and isobutylene
Isobutylene
Isobutylene is a hydrocarbon of significant industrial importance. It is a four-carbon branched alkene , one of the four isomers of butylene. At standard temperature and pressure it is a colorless flammable gas.-Uses:...
.

Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s, as well as tert-butyl acetate, also successfully react with nitriles in the presence of strong acids to form amides via the Ritter reaction.
History
The Ritter reaction is named after John J. Ritter, an American chemist who received his Ph.D.Ph.D.
A Ph.D. is a Doctor of Philosophy, an academic degree.Ph.D. may also refer to:* Ph.D. , a 1980s British group*Piled Higher and Deeper, a web comic strip*PhD: Phantasy Degree, a Korean comic series* PhD Docbook renderer, an XML renderer...
from Columbia University
Columbia University
Columbia University in the City of New York is a private, Ivy League university in Manhattan, New York City. Columbia is the oldest institution of higher learning in the state of New York, the fifth oldest in the United States, and one of the country's nine Colonial Colleges founded before the...
. In 1948, P. Paul Minieri
P. Paul Minieri
P. Paul Minieri, a graduate from New York University, was one of their most successful scientists. Minieri’s contribution to organic chemistry was first established while attending graduate school and working under the guidance of Professor John J. Ritter, he authored a thesis that later became the...
, Ritter's student, submitted work on the reaction as his Ph.D. thesis. Ritter and Minieri collaborated together to perform the experiment in the Microchemistry Laboratory at NYU. Ritter and Minieri described the reaction of a nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
being transformed, in the presence of sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
and alkenes, into an amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
. They characterized the product to confirm that it was a N-alkyl amide through the Kjeldahl method
Kjeldahl method
The Kjeldahl method or Kjeldahl digestion in analytical chemistry is a method for the quantitative determination of nitrogen in chemical substances developed by Johan Kjeldahl in 1883.- Method :...
, which quantitatively determines nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
presence in chemical substances. Although it was developed 62 years ago, the reaction still has significance today due to its applicability and reproducibility of amides via stabilized carbocations.
Reaction mechanism
The Ritter reaction proceeds by the electrophilic addition of either the carbenium ionCarbenium ion
A carbenium ion is a carbocation of the trivalent and classical type R3C+. It is one of two types of carbocation, the other being a carbonium ion. In older literature a carbocation of the type R3C+ may still be referred to as a carbonium ion, a term that is used now for five-coordinate carbon...
2 or covalent species to the nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
. The resulting nitrilium ion 3 is hydrolyzed
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
by water to the desired amide 5.

Applications
The Ritter reaction is most useful in the formation of new carbon-nitrogen bonds, especially in the formation of amides in which the nitrogen has a tertiary alkyl group. It is also used in industrial processes as it can be effectively scaled up from laboratory experiments to large-scale applications while maintaining high yield. Real world applications include MerckMerck
Merck may refer to:* Merck KGaA, , a German-based chemical and pharmaceutical company.** Merck Serono , the pharmaceutical division of Merck KGaA...
’s industrial-scale synthesis of anti-HIV
HIV
Human immunodeficiency virus is a lentivirus that causes acquired immunodeficiency syndrome , a condition in humans in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive...
drug Crixivan (indinavir); the production of the falcipain-2 inhibitor PK 11195; the synthesis of the alkaloid
Alkaloid
Alkaloids are a group of naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Also some synthetic compounds of similar structure are attributed to alkaloids...
aristotelone; and synthesis of Amantadine
Amantadine
Amantadine is the organic compound known formally as 1-adamantylamine or 1-aminoadamantane. The molecule consists of adamantane backbone that has an amino group substituted at one of the four methyne positions. This pharmaceutical is sold under the name Symmetrel for use both as an antiviral and an...
, an antiviral and antiparkinsonian drug. Other applications of the Ritter reaction include synthesis of dopamine receptor
Dopamine receptor
Dopamine receptors are a class of metabotropic G protein-coupled receptors that are prominent in the vertebrate central nervous system . The neurotransmitter dopamine is the primary endogenous ligand for dopamine receptors....
ligands and production of amphetamine
Amphetamine
Amphetamine or amfetamine is a psychostimulant drug of the phenethylamine class which produces increased wakefulness and focus in association with decreased fatigue and appetite.Brand names of medications that contain, or metabolize into, amphetamine include Adderall, Dexedrine, Dextrostat,...
from allylbenzene.
A problem with the Ritter reaction is the necessity of an extremely strong acid catalyst in order to produce the carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
. This poses an environmental risk, as the acids are extremely corrosive and also cannot be reused. However, other methods have been proposed in order promote carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
formation, including photosensitized electron transfer
Electron transfer
Electron transfer is the process by which an electron moves from an atom or a chemical species to another atom or chemical species...
or direct photolysis.

