
Ring-opening polymerization
Encyclopedia
Polymer chemistry
Polymer chemistry or macromolecular chemistry is a multidisciplinary science that deals with the chemical synthesis and chemical properties of polymers or macromolecules. According to IUPAC recommendations, macromolecules refer to the individual molecular chains and are the domain of chemistry...
, ring-opening polymerization is a form of chain-growth polymerization, in which the terminal end of a polymer acts as a reactive center
Reactive center
A reactive center in chemistry is a particular location, usually an atom, within a chemical compound that is the likely center of a reaction in which the chemical is involved. In chain-growth polymer chemistry this is also the point of propagation for a growing chain. The reactive center is...
, where further cyclic monomers
Cyclic compound
In chemistry, a cyclic compound is a compound in which a series of atoms is connected to form a loop or ring.While the vast majority of cyclic compounds are organic, a few inorganic substances form cyclic compounds as well, including sulfur, silanes, phosphanes, phosphoric acid, and triboric acid. ...
join to form a larger polymer chain through ionic propagation. The treatment of some cyclic compounds with catalysts brings about cleavage of the ring followed by polymerization to yield high-molecular-weight polymers. Exemplary polymers produced by this method include:
- Some PolyamidePolyamideA polyamide is a polymer containing monomers of amides joined by peptide bonds. They can occur both naturally and artificially, examples being proteins, such as wool and silk, and can be made artificially through step-growth polymerization or solid-phase synthesis, examples being nylons, aramids,...
s from lactamLactamA lactam is a cyclic amide. Prefixes indicate how many carbon atoms are present in the ring: β-lactam , γ-lactam , δ-lactam...
s:- PA 6Nylon 6Nylon 6 or polycaprolactam is a polymer developed by Paul Schlack at IG Farben to reproduce the properties of nylon 6,6 without violating the patent on its production. Unlike most other nylons, nylon 6 is not a condensation polymer, but instead is formed by ring-opening polymerization. This makes...
: Polycaprolactame from caprolactamCaprolactamCaprolactam is an organic compound with the formula 5CNH. This colourless solid is a lactam or a cyclic amide of caproic acid. Approximately 2 billion kilograms are produced annually... - PA 12: Polylauroamide from lauryllactam
- PA 6
- Some PolyesterPolyesterPolyester is a category of polymers which contain the ester functional group in their main chain. Although there are many polyesters, the term "polyester" as a specific material most commonly refers to polyethylene terephthalate...
s from lactoneLactoneIn chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
s:- PCL : PolycaprolactonePolycaprolactonePolycaprolactone is a biodegradable polyester with a low melting point of around 60°C and a glass transition temperature of about −60°C. PCL is prepared by ring opening polymerization of ε-caprolactone using a catalyst such as stannous octoate. Recently a wide range of catalysts for the ring...
from caprolactoneCaprolactoneε-Caprolactone or simply caprolactone is a cyclic ester, a member of the lactone family, with a seven-membered ring with the formula 5CO2. This colorless liquid is miscible with most organic solvents...
- PCL : Polycaprolactone
- PolyethersEtherEthers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
from cyclic etherEtherEthers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s:- Polyethylene oxidePolyethylene glycolPolyethylene glycol is a polyether compound with many applications from industrial manufacturing to medicine. It has also been known as polyethylene oxide or polyoxyethylene , depending on its molecular weight, and under the tradename Carbowax.-Available forms:PEG, PEO, or POE refers to an...
from ethylene oxideEthylene oxideEthylene oxide, also called oxirane, is the organic compound with the formula . It is a cyclic ether. This means that it is composed of two alkyl groups attached to an oxygen atom in a cyclic shape . This colorless flammable gas with a faintly sweet odor is the simplest epoxide, a three-membered... - Polypropylene oxidePolypropylene glycolPolypropylene glycol or polypropylene oxide is the polymer of propylene glycol. Chemically it is a polyether. The term polypropylene glycol or PPG is reserved for low to medium range molar mass polymer when the nature of the end-group, which is usually a hydroxyl group, still matters...
from propylene oxidePropylene oxidePropylene oxide is an organic compound with the molecular formula CH3CHCH2O. This colourless volatile liquid is produced on a large scale industrially, its major application being its use for the production of polyether polyols for use in making polyurethane plastics... - PolytetrahydrofuranPolytetrahydrofuranPolytetrahydrofuran, also called poly glycol or poly, is a chemical compound with formula n2 or HO-n-OH...
from tetrahydrofuranTetrahydrofuranTetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
- Polyethylene oxide
When the reactive center of the propagating chain is a carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
, the polymerization is called cationic ring-opening polymerization.
When the active center is a carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
, the reaction is an anionic ring-opening polymerization. This is the case of the polypropylene oxide.
A different type of ring-opening polymerization, based on olefin metathesis
Olefin metathesis
Olefin metathesis or transalkylidenation is an organic reaction that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds in olefins . Its advantages include the creation of fewer sideproducts and hazardous wastes. Yves Chauvin, Robert H. Grubbs, and Richard R...
, uses catalysts rather than cationic or anionic propagation.
One example of an anionic ring-opening polymerization is the living polymerization
Living polymerization
In polymer chemistry, living polymerization is a form of addition polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is...
of the aziridine
Aziridine
Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group and two methylene groups...
N-methanesulfonyl-2-methylaziridine with the sulfonamide
Sulfonamide (chemistry)
In chemistry, the sulfonamide functional group is -S2-NH2, a sulfonyl group connected to an amine group.A sulfonamide is a compound that contains this group. The general formula is RSO2NH2, where R is some organic group. For example, "methanesulfonamide" is CH3SO2NH2...
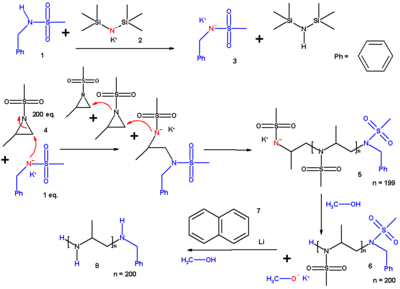
N-benzyl methanesulfonamide (1) and potassium hexamethyldisilazide (2)(Fig. 1). The disilazide actives the anionic initiator
Initiator
An initiator can refer to:* A person that takes an initiative in making something happen.* Modulated neutron initiator, a neutron source used in some nuclear weapons...
by proton exchange which reacts in chain initiation with an aziridine monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
in a ring-opening reaction (DMF
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
, 40°C). In the chain propagation
Chain propagation
Chain propagation is a process in which a reactive intermediate is continuously regenerated during the course of a chemical reaction. In polymerization reaction, the reactive end-groups of a polymer chain react in each propagation step with a new monomer molecule transferring the reactive group to...
step the negatively charged end of the growing polymer chain continues to react with monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
until it is depleted. The reaction is chain terminated
Chain termination
Chain termination is any chemical reaction that ceases the formation of reactive intermediates in a chain propagation step in the course of a polymerization, effectively bringing it to a halt.- Mechanisms of Termination :...
when methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
is added and the active chain end is destroyed. The polymerization is considered living because the polydispersity is low and no evidence is found of branching
Branching (chemistry)
In polymer chemistry, branching occurs by the replacement of a substituent, e.g., a hydrogen atom, on a monomer subunit, by another covalently bonded chain of that polymer; or, in the case of a graft copolymer, by a chain of another type...
. In this particular concept the sulfonyl
Sulfonyl
A sulfonyl group can refer either to a functional group found primarily in sulfones or to a substituent obtained from a sulfonic acid by the removal of the hydroxyl group similarly to acyl groups...
groups can be removed by organic reduction with naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
(7), lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
metal and methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
to the free polyamine
Polyamine
A polyamine is an organic compound having two or more primary amino groups .This class of compounds includes several synthetic substances that are important feedstocks for the chemical industry, such as ethylene diamine , 1,3-diaminopropane , and hexamethylenediamine...
8. This procedure offers an alternative to a cationic ring-opening polymerization of oxazoline
Oxazoline
Oxazoline is both the five-membered ring heterocyclic chemical compound with the formula C3H5NO and the class of compounds containing this ring.- See also :* Desoxazoline * Oxazole* Oxazolidine* Oxazolidinedione...
s with anionic initiators and subsequent acid
Acid catalysis
In acid catalysis and base catalysis a chemical reaction is catalyzed by an acid or a base. The acid is often the proton and the base is often a hydroxyl ion. Typical reactions catalyzed by proton transfer are esterfications and aldol reactions. In these reactions the conjugate acid of the carbonyl...
hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of the acyl
Acyl
An acyl group is a functional group derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids.In organic chemistry, the acyl group is usually derived from a carboxylic acid . Therefore, it has the formula RCO-, where R represents an alkyl group that is...
groups.

