
Sulfonyl
Encyclopedia
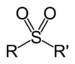
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
found primarily in sulfones or to a substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
obtained from a sulfonic acid
Sulfonic acid
Sulfonic acid usually refers to a member of the class of organosulfur compounds with the general formula RS2–OH, where R is an alkyl or aryl. The formal part of acid, HS2–OH, are formally derivatives of the "parent" inorganic compound with the formula HSO2.-Preparation:Sulfonic acid is...
by the removal of the hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group similarly to acyl groups. Sulfonyl groups can be written as having the general formula R-S(=O)2-R', where there are two double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s between the sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
.
Sulfonyl groups can be reduced to the hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
with lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
(LiAlH4).
In inorganic chemistry
Inorganic chemistry
Inorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds. This field covers all chemical compounds except the myriad organic compounds , which are the subjects of organic chemistry...
, when the group -S(=O)2- is not connected to any carbon atoms, it is referred to as sulfuryl
Sulfuryl
The sulfuryl group is a functional group in inorganic chemistry consisting of a sulfur atom covalently bonded to two oxygen atoms .It occurs in compounds such as sulfuryl chloride, SO2Cl2 and sulfuryl fluoride, SO2F2....
.
Examples of sulfonyl group substituents
The names of sulfonyl groups typically end in -syl, such as:| Group name | Full name | Abbreviation | Example |
|---|---|---|---|
| Tosyl Tosyl A tosyl group is CH3C6H4SO2. This group is usually derived from the compound 4-toluenesulfonyl chloride, CH3C6H4SO2Cl, which forms esters and amides of toluenesulfonic or tosylic acid... |
p-toluenesulfonyl | Ts | Tosyl chloride (p-toluenesulfonyl chloride) CH3C6H4SO2Cl |
| Brosyl | p-bromobenzenesulfonyl | Bs | |
| Nosyl | 2- or 4-nitrobenzenesulfonyl | Ns | |
| Mesyl Mesylate In chemistry, a mesylate is any salt or ester of methanesulfonic acid . In salts, the mesylate is present as the CH3SO3− anion. When modifying the International Nonproprietary Name of a pharmaceutical substance containing the group or anion, the correct spelling is mesilate .Mesylate esters are a... |
methanesulfonyl | Ms | Mesyl chloride (methanesulfonyl chloride) CH3SO2Cl. |
| Triflyl | trifluoromethanesulfonyl | Tf | |
| Dansyl | 5-(dimethylamino)naphthalene-1-sulfonyl | Ds | Dansyl chloride Dansyl chloride Dansyl chloride or 5-naphthalene-1-sulfonyl chloride is a reagent that reacts with primary amino groups in both aliphatic and aromatic amines to produce stable blue- or blue-green–fluorescent sulfonamide adducts. Dansyl chloride is widely used to modify amino acids; specifically, protein sequencing... |

