
Sulfonamide (chemistry)
Encyclopedia
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, the sulfonamide functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
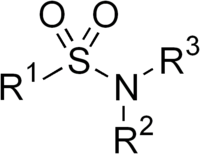
(also spelt sulphonamide ) is -S(=O)2-NH2, a sulfonyl
Sulfonyl
A sulfonyl group can refer either to a functional group found primarily in sulfones or to a substituent obtained from a sulfonic acid by the removal of the hydroxyl group similarly to acyl groups...
group connected to an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
group.
A sulfonamide (compound) is a compound that contains this group. The general formula is RSO2NH2, where R is some organic group. For example, "methanesulfonamide" is CH3SO2NH2. Any sulfonamide can be considered as derived from a sulfonic acid
Sulfonic acid
Sulfonic acid usually refers to a member of the class of organosulfur compounds with the general formula RS2–OH, where R is an alkyl or aryl. The formal part of acid, HS2–OH, are formally derivatives of the "parent" inorganic compound with the formula HSO2.-Preparation:Sulfonic acid is...
by replacing a hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group with an amine group.
In medicine
Medicine
Medicine is the science and art of healing. It encompasses a variety of health care practices evolved to maintain and restore health by the prevention and treatment of illness....
, the term "sulfonamide" is sometimes used as a synonym for sulfa drug
Sulfonamide (medicine)
Sulfonamide or sulphonamide is the basis of several groups of drugs. The original antibacterial sulfonamides are synthetic antimicrobial agents that contain the sulfonamide group. Some sulfonamides are also devoid of antibacterial activity, e.g., the anticonvulsant sultiame...
, a derivative or variation of sulfanilamide.
Organic synthesis
Sulfonamides can be prepared in the laboratory in many ways. For example, by the reaction of sulfonyl chlorides with amineAmine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s in the synthesis of sulfonylmethylamide. A readily available sulfonyl chloride source is tosyl chloride.
Triflimide or triflimidic acid HN(Tf)2 (bis(trifluoromethane)sulfonimide) is the formal adduct of triflic acid and ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
. Phenyl triflimide is a triflating reagent. The related metal triflimidate
Metal triflimidate
A metal triflimidate Mn in organic chemistry is a metal salt of triflimidic acid and used as a catalyst .metal Triflimidates are prepared by reaction of metal oxides, carbonates, hydroxides, or halides with triflimidic acid in water as the hydrate MTfn.xH20 with x ranging from zero to nine...
s are used as catalysts. The anion bistriflimide
Bistriflimide
Bistriflimide, systematically known as bissulfonimide and colloquially known as TFSI is a non-coordinating anion with the chemical formula of [2N]-. The anion is widely used in ionic liquids, since it is less toxic and more stable than more "traditional" counterions such as tetrafluoroborate...
is hydrophobic.
Sulfinamides
The related sulfinamideSulfinamide
Sulfinamide is a functional group in organic chemistry containing a sulfur-oxygen double bond and a sulfur-nitrogen single bond. Sulfinamides are amides of sulfinic acid....
s (R(S=O)NHR) are amides of sulfinic acids (R(S=O)OH) (see sulfinyl). Chiral
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
sulfinamides such as tert-butanesulfinamide
Tert-Butanesulfinamide
tert-Butanesulfinamide is an organosulfur compound and a member of the class of sulfinamides . Both enantiomeric forms are commercially available and are relevant to the asymmetric synthesis of amines as a chiral ammonia equivalent. This methodology was introduced in 1997 by Jonathan A...
, p-toluenesulfinamide and 2,4,6-trimethylbenzenesulfinamide are relevant to asymmetric synthesis.

