Relativistic quantum chemistry
Encyclopedia
Relativistic quantum chemistry invokes quantum chemical
and relativistic mechanical
arguments to explain elemental properties and structure, especially for heavy elements of the periodic table
.
The term "relativistic effects" was developed in light of the history of quantum mechanics. Initially quantum mechanics was developed without considering the theory of relativity
. As per convention, "relativistic effects" are those discrepancies between values calculated by models considering and not considering relativity. "Heavy elements" in this context refers to high atomic number elements in the later part of the periodic table
where relativistic effects are important. Examples are elements found in the lanthanide and actinide series.
Relativistic effects in chemistry can be considered to be perturbations, or small corrections, to the non-relativistic theory of chemistry, which is developed from the solutions of the Schrödinger equation
. These corrections have differential effects on the electrons in various atomic orbital
s within the atom, according to the speed of these electrons relative to the speed of light
. Relativistic effects are more prominent in heavy elements, because only in these elements do electrons attain relativistic speeds.
describes a relativistic treatment of a many-electron system, in spite of Paul Dirac
's 1929 assertion that the only imperfections remaining in quantum mechanics
Theoretical chemists by and large agreed with Dirac's sentiment until the 1970s, when relativistic effects began to become realized in heavy elements. The Schrödinger equation
had been developed without considering relativity in Schrödinger's famous 1926 paper. Relativistic corrections were made to the Schrödinger equation (see Klein-Gordon equation
) in order to explain the fine structure
of atomic spectra, but this development and others did not immediately trickle into the chemical community. Since atomic spectral line
s were largely in the realm of physics and not chemistry, most chemists were unfamiliar with relativistic quantum mechanics, and the focus at the time was on lighter elements typical for the organic chemistry
focus of the time.
Dirac's opinion on the role relativistic quantum mechanics would play for chemical systems is wrong for two reasons: the first being that electrons in s and p atomic orbitals travel at a significant fraction of the speed of light and the second being that there are indirect consequences of relativistic effects which are especially evident for d and f atomic orbital
s.
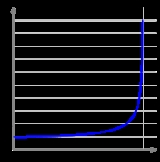
 One of the most important and familiar results of relativity is that the relativistic mass
One of the most important and familiar results of relativity is that the relativistic mass
of the electron
increases by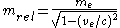
where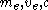 are the electron rest mass
are the electron rest mass
, velocity
of the electron, and speed of light
respectively. The figure at the right illustrates the relativistic effects on the mass of an electron as a function of its velocity.
This has an immediate implication on the Bohr radius
(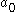 ) which is given by
) which is given by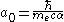
where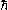 is the reduced Planck's constant and
is the reduced Planck's constant and  is the fine-structure constant
is the fine-structure constant
(a relativistic correction for the Bohr model
).
Arnold Sommerfeld
calculated that, for a 1s electron of a hydrogen atom with an orbiting radius of 0.0529 nm,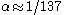 . That is to say, the fine-structure constant shows the electron traveling at nearly 1/137 the speed of light. One can extend this to a larger element by using the expression
. That is to say, the fine-structure constant shows the electron traveling at nearly 1/137 the speed of light. One can extend this to a larger element by using the expression 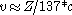 for a 1s electron where v is its radial velocity. For gold with
for a 1s electron where v is its radial velocity. For gold with 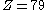 the 1s electron will be going (
the 1s electron will be going (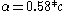 ) 58% of the speed of light. Plugging this in for
) 58% of the speed of light. Plugging this in for 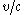 for the relativistic mass one finds that
for the relativistic mass one finds that 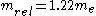 and in turn putting this in for the Bohr radius above one finds that the radius shrinks by 22%.
and in turn putting this in for the Bohr radius above one finds that the radius shrinks by 22%.
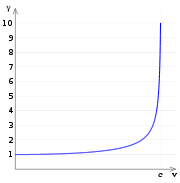
If one substitutes in the relativistic mass into the equation for the Bohr radius it can be written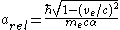
 It follows that
It follows that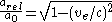
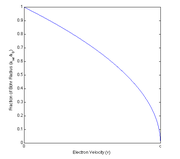
To your right this fraction of the relativistic and unrelativistic Bohr radius has been plotted as a function of the electron velocity. Notice how the relativistic model shows the radius decreasing for an ever-larger velocity.
The same result is obtained when the relativistic effect of length contraction
is applied to the radius of the 6s orbital. The length contraction is expressed as
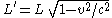
so the radius of the 6s orbital shrinks to
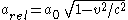
which is consistent with the result obtained by incorporating the increase of mass.
When the Bohr treatment is extended to hydrogenic-like atoms
using the Quantum Rule, the Bohr radius becomes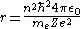
where is the principal quantum number
is the principal quantum number
and Z is an integer for the atomic number
. From quantum mechanics the angular momentum
is given as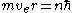 . Substituting into the equation above and solving for
. Substituting into the equation above and solving for  gives
gives
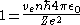
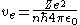
From this point atomic units
can be used to simplify the expression into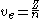
Substituting this into the expression for the Bohr ratio mentioned above gives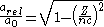
At this point one can see that for a low value of and a high value of
and a high value of 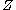 that
that 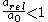 . This fits with intuition: electrons with lower principal quantum numbers will have a higher probability density of being nearer to the nucleus. A nucleus with a large charge will cause an electron to have a high velocity. A higher electron velocity means an increased electron relativistic mass, as a result the electrons will be near the nucleus more of the time and thereby contract the radius for small principal quantum numbers.
. This fits with intuition: electrons with lower principal quantum numbers will have a higher probability density of being nearer to the nucleus. A nucleus with a large charge will cause an electron to have a high velocity. A higher electron velocity means an increased electron relativistic mass, as a result the electrons will be near the nucleus more of the time and thereby contract the radius for small principal quantum numbers.
was constructed by scientists who noticed periodic trends in known elements of the time. Indeed, the patterns found in it is what gives the Periodic table its power. Many of the chemical and physical differences between the 6th Row (Cs-Rn) and the 5th Row (Rb-Xe) arise from the larger relativistic effects for the former. These relativistic effects are particularly large for gold and its neighbors, platinum and mercury.
(°C) (see m.p.
). Bonding forces are weaker for Hg-Hg bonds than for its immediate neighbors such as cadmium
(m.p. 321 °C) and gold
(m.p. 1064 °C). The lanthanide contraction
is a partial explanation, however, it does not entirely account for this anomaly. In the gas phase mercury is alone in metals in that it is quite typically found in a monomeric form as Hg(g). Hg22+(g) also forms and it is a stable species due to the relativistic shortening of the bond.
Hg2(g) does not form because the 6s2 orbital is contracted by relativistic effects and may therefore only weakly contribute to any bonding; in fact Hg-Hg bonding must be mostly the result of van der Waals forces which explains why the bonding for Hg-Hg is weak enough to allow for Hg to be a liquid at room temperature.
Au2(g) and Hg(g) are analogous, at the least in having the same nature of difference, to H2(g) and He(g). It is for the relativistic contraction of the 6s2 orbital that gaseous mercury can be called pseudo noble gas.
 The reflectivity
The reflectivity
of Au, Ag, Al is shown on the figure to the right. The human eye sees electromagnetic radiation with a wavelength near 600 nm as yellow. As is clear from the spectral reflectance curves for Au, the reason for seeing it yellow is that it is absorbing
all the radiation not necessary for yellow and reflecting those wavelengths necessary to see yellow at an observer.
The electronic transition responsible for this absorption is a transition from the 5d to the 6s level. An analogous transition occurs in Ag but the relativistic effects are lower in Ag so while the 4d experiences some expansion and the 5s some contraction, the 4d-5s distance in Ag is still much greater than the 5d-6s distance in Au because the relativistic effects in Ag are smaller than those in Au. Thus, nonrelativistic gold would be white. The relativistic effects are raising the 5d orbital and lowering the 6s orbital.
A similar effect occurs in caesium
metal, the heaviest of the alkali metals which can be collected in quantities sufficient to allow viewing. Whereas the other alkaline metals are silver-white, caesium metal has a distinctly golden hue.
), Pb(II) (lead
), and Bi(III) (bismuth
) complexes
there is a 6s2 electron pair. The 'inert pair effect' refers to the tendency for this pair of electrons to resist oxidation due to a relativistic contraction of the 6s orbital.
Quantum chemistry
Quantum chemistry is a branch of chemistry whose primary focus is the application of quantum mechanics in physical models and experiments of chemical systems...
and relativistic mechanical
Theory of relativity
The theory of relativity, or simply relativity, encompasses two theories of Albert Einstein: special relativity and general relativity. However, the word relativity is sometimes used in reference to Galilean invariance....
arguments to explain elemental properties and structure, especially for heavy elements of the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
.
The term "relativistic effects" was developed in light of the history of quantum mechanics. Initially quantum mechanics was developed without considering the theory of relativity
Theory of relativity
The theory of relativity, or simply relativity, encompasses two theories of Albert Einstein: special relativity and general relativity. However, the word relativity is sometimes used in reference to Galilean invariance....
. As per convention, "relativistic effects" are those discrepancies between values calculated by models considering and not considering relativity. "Heavy elements" in this context refers to high atomic number elements in the later part of the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
where relativistic effects are important. Examples are elements found in the lanthanide and actinide series.
Relativistic effects in chemistry can be considered to be perturbations, or small corrections, to the non-relativistic theory of chemistry, which is developed from the solutions of the Schrödinger equation
Schrödinger equation
The Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
. These corrections have differential effects on the electrons in various atomic orbital
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
s within the atom, according to the speed of these electrons relative to the speed of light
Speed of light
The speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
. Relativistic effects are more prominent in heavy elements, because only in these elements do electrons attain relativistic speeds.
History
Beginning in 1935 Bertha SwirlesBertha Swirles
Bertha Swirles, Lady Jeffreys , carried out research on quantum theory, particularly in its early days. She was associated with Girton College, University of Cambridge, as student and Fellow, for over 70 years....
describes a relativistic treatment of a many-electron system, in spite of Paul Dirac
Paul Dirac
Paul Adrien Maurice Dirac, OM, FRS was an English theoretical physicist who made fundamental contributions to the early development of both quantum mechanics and quantum electrodynamics...
's 1929 assertion that the only imperfections remaining in quantum mechanics
Theoretical chemists by and large agreed with Dirac's sentiment until the 1970s, when relativistic effects began to become realized in heavy elements. The Schrödinger equation
Schrödinger equation
The Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
had been developed without considering relativity in Schrödinger's famous 1926 paper. Relativistic corrections were made to the Schrödinger equation (see Klein-Gordon equation
Klein-Gordon equation
The Klein–Gordon equation is a relativistic version of the Schrödinger equation....
) in order to explain the fine structure
Fine structure
In atomic physics, the fine structure describes the splitting of the spectral lines of atoms due to first order relativistic corrections.The gross structure of line spectra is the line spectra predicted by non-relativistic electrons with no spin. For a hydrogenic atom, the gross structure energy...
of atomic spectra, but this development and others did not immediately trickle into the chemical community. Since atomic spectral line
Atomic spectral line
In physics, atomic spectral lines are of two types:* An emission line is formed when an electron makes a transition from a particular discrete energy level of an atom, to a lower energy state, emitting a photon of a particular energy and wavelength...
s were largely in the realm of physics and not chemistry, most chemists were unfamiliar with relativistic quantum mechanics, and the focus at the time was on lighter elements typical for the organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
focus of the time.
Dirac's opinion on the role relativistic quantum mechanics would play for chemical systems is wrong for two reasons: the first being that electrons in s and p atomic orbitals travel at a significant fraction of the speed of light and the second being that there are indirect consequences of relativistic effects which are especially evident for d and f atomic orbital
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
s.
Qualitative treatment

Mass in special relativity
Mass in special relativity incorporates the general understandings from the concept of mass-energy equivalence. Added to this concept is an additional complication resulting from the fact that "mass" is defined in two different ways in special relativity: one way defines mass as an invariant...
of the electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
increases by
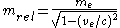
where
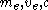 are the electron rest mass
are the electron rest massElectron rest mass
The electron rest mass is the mass of a stationary electron. It is one of the fundamental constants of physics, and is also very important in chemistry because of its relation to the Avogadro constant...
, velocity
Velocity
In physics, velocity is speed in a given direction. Speed describes only how fast an object is moving, whereas velocity gives both the speed and direction of the object's motion. To have a constant velocity, an object must have a constant speed and motion in a constant direction. Constant ...
of the electron, and speed of light
Speed of light
The speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
respectively. The figure at the right illustrates the relativistic effects on the mass of an electron as a function of its velocity.
This has an immediate implication on the Bohr radius
Bohr radius
The Bohr radius is a physical constant, approximately equal to the most probable distance between the proton and electron in a hydrogen atom in its ground state. It is named after Niels Bohr, due to its role in the Bohr model of an atom...
(
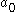 ) which is given by
) which is given by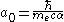
where
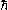 is the reduced Planck's constant and
is the reduced Planck's constant and  is the fine-structure constant
is the fine-structure constantFine-structure constant
In physics, the fine-structure constant is a fundamental physical constant, namely the coupling constant characterizing the strength of the electromagnetic interaction. Being a dimensionless quantity, it has constant numerical value in all systems of units...
(a relativistic correction for the Bohr model
Bohr model
In atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
).
Arnold Sommerfeld
Arnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld was a German theoretical physicist who pioneered developments in atomic and quantum physics, and also educated and groomed a large number of students for the new era of theoretical physics...
calculated that, for a 1s electron of a hydrogen atom with an orbiting radius of 0.0529 nm,
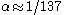 . That is to say, the fine-structure constant shows the electron traveling at nearly 1/137 the speed of light. One can extend this to a larger element by using the expression
. That is to say, the fine-structure constant shows the electron traveling at nearly 1/137 the speed of light. One can extend this to a larger element by using the expression 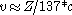 for a 1s electron where v is its radial velocity. For gold with
for a 1s electron where v is its radial velocity. For gold with 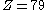 the 1s electron will be going (
the 1s electron will be going (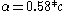 ) 58% of the speed of light. Plugging this in for
) 58% of the speed of light. Plugging this in for 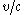 for the relativistic mass one finds that
for the relativistic mass one finds that 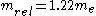 and in turn putting this in for the Bohr radius above one finds that the radius shrinks by 22%.
and in turn putting this in for the Bohr radius above one finds that the radius shrinks by 22%.If one substitutes in the relativistic mass into the equation for the Bohr radius it can be written
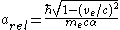

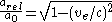
To your right this fraction of the relativistic and unrelativistic Bohr radius has been plotted as a function of the electron velocity. Notice how the relativistic model shows the radius decreasing for an ever-larger velocity.
The same result is obtained when the relativistic effect of length contraction
Length contraction
In physics, length contraction – according to Hendrik Lorentz – is the physical phenomenon of a decrease in length detected by an observer of objects that travel at any non-zero velocity relative to that observer...
is applied to the radius of the 6s orbital. The length contraction is expressed as
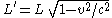
so the radius of the 6s orbital shrinks to
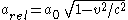
which is consistent with the result obtained by incorporating the increase of mass.
When the Bohr treatment is extended to hydrogenic-like atoms
Hydrogen-like atom
A hydrogen-like ion is any atomic nucleus with one electron and thus is isoelectronic with hydrogen. Except for the hydrogen atom itself , these ions carry the positive charge e, where Z is the atomic number of the atom. Examples of hydrogen-like ions are He+, Li2+, Be3+ and B4+...
using the Quantum Rule, the Bohr radius becomes
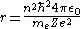
where
 is the principal quantum number
is the principal quantum numberPrincipal quantum number
In atomic physics, the principal quantum symbolized as n is the firstof a set of quantum numbers of an atomic orbital. The principal quantum number can only have positive integer values...
and Z is an integer for the atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
. From quantum mechanics the angular momentum
Angular momentum
In physics, angular momentum, moment of momentum, or rotational momentum is a conserved vector quantity that can be used to describe the overall state of a physical system...
is given as
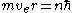 . Substituting into the equation above and solving for
. Substituting into the equation above and solving for  gives
gives
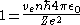
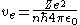
From this point atomic units
Atomic units
Atomic units form a system of natural units which is especially convenient for atomic physics calculations. There are two different kinds of atomic units, which one might name Hartree atomic units and Rydberg atomic units, which differ in the choice of the unit of mass and charge. This article...
can be used to simplify the expression into
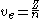
Substituting this into the expression for the Bohr ratio mentioned above gives
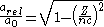
At this point one can see that for a low value of
 and a high value of
and a high value of 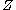 that
that 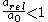 . This fits with intuition: electrons with lower principal quantum numbers will have a higher probability density of being nearer to the nucleus. A nucleus with a large charge will cause an electron to have a high velocity. A higher electron velocity means an increased electron relativistic mass, as a result the electrons will be near the nucleus more of the time and thereby contract the radius for small principal quantum numbers.
. This fits with intuition: electrons with lower principal quantum numbers will have a higher probability density of being nearer to the nucleus. A nucleus with a large charge will cause an electron to have a high velocity. A higher electron velocity means an increased electron relativistic mass, as a result the electrons will be near the nucleus more of the time and thereby contract the radius for small principal quantum numbers.Periodic table deviations
The Periodic tablePeriodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
was constructed by scientists who noticed periodic trends in known elements of the time. Indeed, the patterns found in it is what gives the Periodic table its power. Many of the chemical and physical differences between the 6th Row (Cs-Rn) and the 5th Row (Rb-Xe) arise from the larger relativistic effects for the former. These relativistic effects are particularly large for gold and its neighbors, platinum and mercury.
Mercury
Mercury (Hg) is a liquid down to -39 °CelsiusCelsius
Celsius is a scale and unit of measurement for temperature. It is named after the Swedish astronomer Anders Celsius , who developed a similar temperature scale two years before his death...
(°C) (see m.p.
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
). Bonding forces are weaker for Hg-Hg bonds than for its immediate neighbors such as cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
(m.p. 321 °C) and gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
(m.p. 1064 °C). The lanthanide contraction
Lanthanide contraction
Lanthanide contraction is a term used in chemistry to describe the decrease in ionic radii of the elements in the lanthanide series from atomic number 58, Cerium to 71, Lutetium, which results in smaller than otherwise expected ionic radii for the subsequent elements starting with 72, Hafnium...
is a partial explanation, however, it does not entirely account for this anomaly. In the gas phase mercury is alone in metals in that it is quite typically found in a monomeric form as Hg(g). Hg22+(g) also forms and it is a stable species due to the relativistic shortening of the bond.
Hg2(g) does not form because the 6s2 orbital is contracted by relativistic effects and may therefore only weakly contribute to any bonding; in fact Hg-Hg bonding must be mostly the result of van der Waals forces which explains why the bonding for Hg-Hg is weak enough to allow for Hg to be a liquid at room temperature.
Au2(g) and Hg(g) are analogous, at the least in having the same nature of difference, to H2(g) and He(g). It is for the relativistic contraction of the 6s2 orbital that gaseous mercury can be called pseudo noble gas.
Colour of gold and caesium

Reflectivity
In optics and photometry, reflectivity is the fraction of incident radiation reflected by a surface. In general it must be treated as a directional property that is a function of the reflected direction, the incident direction, and the incident wavelength...
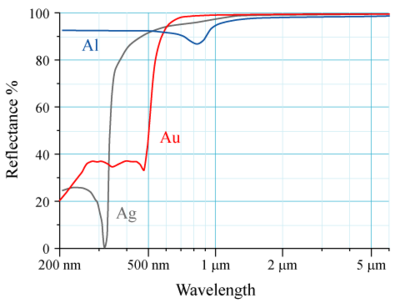
of Au, Ag, Al is shown on the figure to the right. The human eye sees electromagnetic radiation with a wavelength near 600 nm as yellow. As is clear from the spectral reflectance curves for Au, the reason for seeing it yellow is that it is absorbing
Absorption (electromagnetic radiation)
In physics, absorption of electromagnetic radiation is the way by which the energy of a photon is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed to other forms of energy for example, to heat. The absorption of light during wave propagation is...
all the radiation not necessary for yellow and reflecting those wavelengths necessary to see yellow at an observer.
The electronic transition responsible for this absorption is a transition from the 5d to the 6s level. An analogous transition occurs in Ag but the relativistic effects are lower in Ag so while the 4d experiences some expansion and the 5s some contraction, the 4d-5s distance in Ag is still much greater than the 5d-6s distance in Au because the relativistic effects in Ag are smaller than those in Au. Thus, nonrelativistic gold would be white. The relativistic effects are raising the 5d orbital and lowering the 6s orbital.
A similar effect occurs in caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
metal, the heaviest of the alkali metals which can be collected in quantities sufficient to allow viewing. Whereas the other alkaline metals are silver-white, caesium metal has a distinctly golden hue.
Inert pair effect
In Tl(I) (thalliumThallium
Thallium is a chemical element with the symbol Tl and atomic number 81. This soft gray poor metal resembles tin but discolors when exposed to air. The two chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861 by the newly developed method of flame spectroscopy...
), Pb(II) (lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
), and Bi(III) (bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
) complexes
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
there is a 6s2 electron pair. The 'inert pair effect' refers to the tendency for this pair of electrons to resist oxidation due to a relativistic contraction of the 6s orbital.
Others
Some of the phenomena commonly attributed to relativistic effects are:- The stability of mercury(IV) fluorideMercury(IV) fluorideMercury fluoride, HgF4, is the first mercury compound to be discovered with the metal in the oxidation state IV. Mercury, like the other group 12 elements , has an s2d10 electron configuration and generally only forms bonds involving its s orbital...
- AurophilicityAurophilicityIn chemistry, aurophilicity refers to the tendency of gold complexes to aggregate via formation of weak gold-gold bonds.-Overview:The phenomenon of aurophilicity is most commonly observed crystallographically for Au compounds. The aurophilic bond has a length of about 3.0 Å and a strength of about...
- The stability of the gold anion, Au−, in compounds such as CsAu
- The crystal structure of leadLeadLead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
, which is face-centered cubic instead of diamond-like - The striking similarity between zirconiumZirconiumZirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
and hafniumHafniumHafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869. Hafnium was the penultimate stable... - The stability of the uranyl cation, as well as other high oxidation states in the early actinideActinideThe actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
s (Pa-Am) - The small atomic radii of franciumFranciumFrancium is a chemical element with symbol Fr and atomic number 87. It was formerly known as eka-caesium and actinium K.Actually the least unstable isotope, francium-223 It has the lowest electronegativity of all known elements, and is the second rarest naturally occurring element...
and radiumRadiumRadium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,... - About 10% of the lanthanide contractionLanthanide contractionLanthanide contraction is a term used in chemistry to describe the decrease in ionic radii of the elements in the lanthanide series from atomic number 58, Cerium to 71, Lutetium, which results in smaller than otherwise expected ionic radii for the subsequent elements starting with 72, Hafnium...
is attributed to relativistic effects
Further reading
- P. A. Christiansen; W. C. Ermler; K. S. Pitzer. Relativistic Effects in Chemical Systems. Annual Review of Physical Chemistry 1985, 36, 407-432.
- Pekka Pyykko. Relativistic effects in structural chemistry. Chem. Rev. 1988, 88, 563-594.

