
Pyramidal alkene
Encyclopedia
Pyramidal alkenes are alkene
s in which the two carbon
atom
s making up the double bond
are not coplanar with their four substituent
s . This deformation from a trigonal planar
geometry to a tetrahedral molecular geometry
is the result of angle strain
induced in the molecule due to geometric constraints. Pyramidal alkenes only exist in the laboratory but are of interest because much can be learned from them about the nature of chemical bond
ing.
In cycloheptene
(1.1) the cis isomer is an ordinary unstrained molecule but the heptane ring is too small to accommodate a trans configured alkene group resulting in strain and twisting of the double bond. The p-orbital misalignment is minimized by a degree of pyramidalization. In the related anti-bredt molecules it is not pyrimidalization but twisting that dominates.
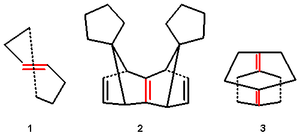 Pyramidalized cage alkenes also exist where symmetrical bending of the substituents predominates without p-orbital misalignment.
Pyramidalized cage alkenes also exist where symmetrical bending of the substituents predominates without p-orbital misalignment.
 The pyramidalization angle
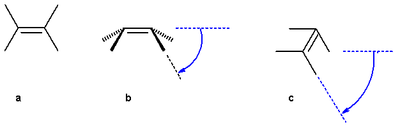
The pyramidalization angle  (b) is defined as the angle between the plane defined by one of the doubly bonded carbons and its two substituents and the extension of the double bond and is calculated as:
(b) is defined as the angle between the plane defined by one of the doubly bonded carbons and its two substituents and the extension of the double bond and is calculated as:
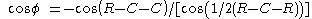
the butterfly bending angle or folding angle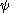 (c) is defined as the angle between two planes and can be obtained by averaging the two torsional angles R1C:::CR3 and R2C:::CR4.
(c) is defined as the angle between two planes and can be obtained by averaging the two torsional angles R1C:::CR3 and R2C:::CR4.
In alkenes 1.2 and 1.3 these angles are determined with x-ray crystallography
as respectively 32.4°/22.7° and 27.3°/35.6°. Although stable, these alkenes are very reactive compared to ordinary alkenes. They are liable to dimerization to cyclobutadiene
compounds or react with oxygen to epoxide
s.
The compound tetradehydrodianthracene also with a 35° pyramidalization angle is synthesized in a photochemical cycloaddition
of bromoanthracene
followed by elimination
of hydrogen bromide
 This compound is very reactive in Diels-Alder reaction
This compound is very reactive in Diels-Alder reaction
s due to through-space interactions between the two alken groups. This enhanced reactivity enabled in turn the synthesis of the first-ever Möbius aromat
.
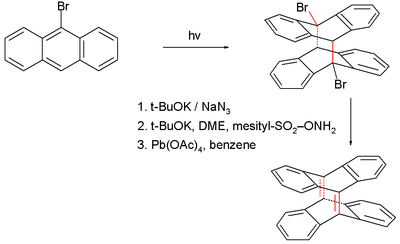
In one study the strained alkene 3.4 was synthesized with the highest pyramidalizion angles yet, 33.5° and 34.3°. This compound is the double Diels-Alder adduct of the diiodide-cyclophane
3.1 and anthracene
3.3 by reaction in presence of potassium tert-butoxide
in reflux
ing dibutyl ether through a di-aryne
intermediate 3.2. This is a stable compound but will slowly react with oxygen to an epoxide
when left standing as a chloroform
solution.
 In one study, isolation of a pyramidal alkene is not even possible in by matrix isolation
In one study, isolation of a pyramidal alkene is not even possible in by matrix isolation
at extremely low temperatures unless stabilized by metal coordination:
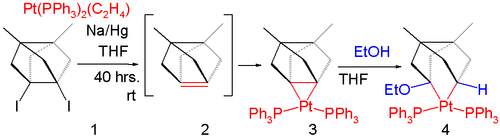 A reaction of the di-iodide
A reaction of the di-iodide
4.1 in fig. 4 with sodium amalgam
in the presence of ethylenebis(triphenylphosphine)platinum(0) does not give the intermediate alkene
4.2 but the platinum stabilized 4.3. The sigma bond in this compound is destroyed in reaction with ethanol
.
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s in which the two carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s making up the double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
are not coplanar with their four substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s . This deformation from a trigonal planar
Trigonal planar
In chemistry, trigonal planar is a molecular geometry model with one atom at the center and three atoms at the corners of a triangle, called peripheral atoms, all in one plane. In an ideal trigonal planar species, all three ligands are identical and all bond angles are 120°. Such species belong to...
geometry to a tetrahedral molecular geometry
Tetrahedral molecular geometry
In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1 ≈ 109.5° when all four substituents are the same, as in CH4. This molecular geometry is common throughout the first...
is the result of angle strain
Angle strain
Angle strain, also called Baeyer strain in cyclic molecules, is the resistance associated with bond angle compression or bond angle expansion. It occurs when bond angles deviate from the ideal bond angles to achieve maximum bond strength in a specific chemical conformation...
induced in the molecule due to geometric constraints. Pyramidal alkenes only exist in the laboratory but are of interest because much can be learned from them about the nature of chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
ing.
In cycloheptene
Cycloheptene
Cycloheptene is a 7-membered cycloalkene with a flash point of -6 C°. It is a raw material in organic chemistry and a monomer in polymer synthesis. Cycloheptene can exist as either the cis- or the trans-isomer.- trans-Cycloheptene :...
(1.1) the cis isomer is an ordinary unstrained molecule but the heptane ring is too small to accommodate a trans configured alkene group resulting in strain and twisting of the double bond. The p-orbital misalignment is minimized by a degree of pyramidalization. In the related anti-bredt molecules it is not pyrimidalization but twisting that dominates.


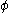 (b) is defined as the angle between the plane defined by one of the doubly bonded carbons and its two substituents and the extension of the double bond and is calculated as:
(b) is defined as the angle between the plane defined by one of the doubly bonded carbons and its two substituents and the extension of the double bond and is calculated as: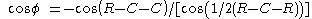
the butterfly bending angle or folding angle
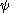 (c) is defined as the angle between two planes and can be obtained by averaging the two torsional angles R1C:::CR3 and R2C:::CR4.
(c) is defined as the angle between two planes and can be obtained by averaging the two torsional angles R1C:::CR3 and R2C:::CR4.In alkenes 1.2 and 1.3 these angles are determined with x-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
as respectively 32.4°/22.7° and 27.3°/35.6°. Although stable, these alkenes are very reactive compared to ordinary alkenes. They are liable to dimerization to cyclobutadiene
Cyclobutadiene
Cyclobutadiene is the smallest [n]-annulene , an extremely unstable hydrocarbon having a lifetime shorter than five seconds in the free state. It has chemical formula 44 and a rectangular structure verified by infrared studies. This is in contrast to the square geometry predicted by simple Hückel...
compounds or react with oxygen to epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
s.
The compound tetradehydrodianthracene also with a 35° pyramidalization angle is synthesized in a photochemical cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
of bromoanthracene
Anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon consisting of three fused benzene rings. It is a component of coal-tar. Anthracene is used in the production of the red dye alizarin and other dyes...
followed by elimination
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
of hydrogen bromide
Hydrogen bromide
Hydrogen bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation. Hydrogen bromide and...

Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
s due to through-space interactions between the two alken groups. This enhanced reactivity enabled in turn the synthesis of the first-ever Möbius aromat
Möbius aromaticity
In organic chemistry, Möbius aromaticity is a special type of aromaticity believed to exist in a number of organic molecules. In terms of MO theory these compounds have in common a monocyclic array of molecular orbitals in which there is an odd number of out-of-phase overlaps which reveals the...
.
In one study the strained alkene 3.4 was synthesized with the highest pyramidalizion angles yet, 33.5° and 34.3°. This compound is the double Diels-Alder adduct of the diiodide-cyclophane
Cyclophane
A cyclophane is a hydrocarbon consisting of an aromatic unit and an aliphatic chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known...
3.1 and anthracene
Anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon consisting of three fused benzene rings. It is a component of coal-tar. Anthracene is used in the production of the red dye alizarin and other dyes...
3.3 by reaction in presence of potassium tert-butoxide
Potassium tert-butoxide
Potassium tert-butoxide is the chemical compound with the formula 3COK. This colourless solid is a strong base useful in organic synthesis. It exists as a tetrameric cubane-like cluster...
in reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
ing dibutyl ether through a di-aryne
Aryne
In chemistry, an aryne is an uncharged reactive intermediate derived from an aromatic system by removal of two ortho substituents, leaving two orbitals with two electrons distributed between them....
intermediate 3.2. This is a stable compound but will slowly react with oxygen to an epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
when left standing as a chloroform
Chloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
solution.

Matrix Isolation
Matrix isolation is an experimental technique used in chemistry and physics which generally involves a material being trapped within an unreactive matrix. A host matrix is a continuous solid phase in which guest particles are embedded. The guest is said to be isolated within the host matrix...
at extremely low temperatures unless stabilized by metal coordination:

Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
4.1 in fig. 4 with sodium amalgam
Sodium amalgam
Sodium amalgam, commonly denoted Na, is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions involving mercury as a major component. Sodium amalgam is often used in reactions as strong reducing agents with better handling properties compared...
in the presence of ethylenebis(triphenylphosphine)platinum(0) does not give the intermediate alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
4.2 but the platinum stabilized 4.3. The sigma bond in this compound is destroyed in reaction with ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
.

