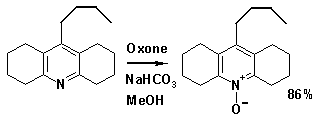Potassium peroxymonosulfate
Encyclopedia
Potassium peroxymonosulfate (also known as MPS
, potassium monopersulfate, and the trade names Caroat and Oxone) is widely used as an oxidizing agent
. It is the potassium
salt of peroxymonosulfuric acid
.
The potassium salt is marketed by two companies: Evonik (formerly Degussa) under the tradename Caroat and DuPont
under the tradename Oxone, tradenames which are now part of standard chemistry vocabulary. It is a component of a triple salt with the formula 2KHSO5·KHSO4·K2SO4. The standard electrode potential
for this compound is +1.44 V with a half reaction generating the hydrogen sulfate.
s to carboxylic acid
s; in the presence of alcoholic solvents, the ester
s may be obtained. Internal alkene
s may be cleaved to two carboxylic acid
s, while terminal alkenes may be epoxidized
. Thioether
s give sulfone
s, tertiary amine
s give amine oxide
s, and phosphine
s give phosphine oxide
s.
Illustrative of the oxidation power of this salt is the conversion of an acridine
derivative to the corresponding acridine-N-oxide.
It will also oxidize a thioether
to a sulfone
with 2 equivalents. With one equivalent the reaction converting sulfide to sulfoxide is much faster than that of sulfoxide to sulfone, so the reaction can conveniently be stopped at that stage if so desired.
s to keep the water clear, thus allowing chlorine in pools to work to sanitize the water rather than clarify the water, resulting in less chlorine needed to keep pools clean. One of the drawbacks of using potassium peroxymonosulfate in pools is it can cause the common DPD #3 water test for combined chlorine to read incorrectly high.
Technical
MPS
MPS may refer to:* Robinson List, aka Mail Preference Service, direct mail opt-out system* Malmin Palloseura, association football club from Helsinki, Finland.* Marginal propensity to save* Master Production Schedule...
, potassium monopersulfate, and the trade names Caroat and Oxone) is widely used as an oxidizing agent
Oxidizing agent
An oxidizing agent can be defined as a substance that removes electrons from another reactant in a redox chemical reaction...
. It is the potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
salt of peroxymonosulfuric acid
Peroxymonosulfuric acid
Peroxymonosulfuric acid, also known as persulfuric acid, peroxysulfuric acid, or as Caro's acid, is H2SO5, a liquid at room temperature. In this acid, the S center adopts its characteristic tetrahedral geometry; the connectivity is indicated by the formula HO-O-S2-OH...
.
The potassium salt is marketed by two companies: Evonik (formerly Degussa) under the tradename Caroat and DuPont
DuPont
E. I. du Pont de Nemours and Company , commonly referred to as DuPont, is an American chemical company that was founded in July 1802 as a gunpowder mill by Eleuthère Irénée du Pont. DuPont was the world's third largest chemical company based on market capitalization and ninth based on revenue in 2009...
under the tradename Oxone, tradenames which are now part of standard chemistry vocabulary. It is a component of a triple salt with the formula 2KHSO5·KHSO4·K2SO4. The standard electrode potential
Standard electrode potential
In electrochemistry, the standard electrode potential, abbreviated E° or E , is the measure of individual potential of a reversible electrode at standard state, which is with solutes at an effective concentration of 1 mol dm−3, and gases at a pressure of 1 atm...
for this compound is +1.44 V with a half reaction generating the hydrogen sulfate.
-
- HSO5− + 2 H+ + 2 e− → HSO4− + H2O
Reactions
Oxone is a versatile oxidant. It oxidizes aldehydeAldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s to carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s; in the presence of alcoholic solvents, the ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s may be obtained. Internal alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s may be cleaved to two carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s, while terminal alkenes may be epoxidized
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
. Thioether
Thioether
A thioether is a functional group in organosulfur chemistry with the connectivity C-S-C as shown on right. Like many other sulfur-containing compounds, volatile thioethers have foul odors. A thioether is similar to an ether except that it contains a sulfur atom in place of the oxygen...
s give sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
s, tertiary amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s give amine oxide
Amine oxide
An amine oxide, also known as amine-N-oxide and N-oxide, is a chemical compound that contains the functional group R3N+-O−, an N-O bond with three additional hydrogen and/or hydrocarbon side chains attached to N. Sometimes it is written as R3N→O or, wrongly, as R3N=O.In the strict sense the...
s, and phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
s give phosphine oxide
Phosphine oxide
Phosphine oxides are either inorganic phosphorus compounds such as phosphoryl trichloride or organophosphorus compounds with the formula OPR3, where R = alkyl or aryl...
s.
Illustrative of the oxidation power of this salt is the conversion of an acridine
Acridine
Acridine, C13H9N, is an organic compound and a nitrogen heterocycle. Acridine is also used to describe compounds containing the C13N tricycle....
derivative to the corresponding acridine-N-oxide.
It will also oxidize a thioether
Thioether
A thioether is a functional group in organosulfur chemistry with the connectivity C-S-C as shown on right. Like many other sulfur-containing compounds, volatile thioethers have foul odors. A thioether is similar to an ether except that it contains a sulfur atom in place of the oxygen...
to a sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
with 2 equivalents. With one equivalent the reaction converting sulfide to sulfoxide is much faster than that of sulfoxide to sulfone, so the reaction can conveniently be stopped at that stage if so desired.
Uses
Potassium peroxymonosulfate can be used in swimming poolSwimming pool
A swimming pool, swimming bath, wading pool, or simply a pool, is a container filled with water intended for swimming or water-based recreation. There are many standard sizes; the largest is the Olympic-size swimming pool...
s to keep the water clear, thus allowing chlorine in pools to work to sanitize the water rather than clarify the water, resulting in less chlorine needed to keep pools clean. One of the drawbacks of using potassium peroxymonosulfate in pools is it can cause the common DPD #3 water test for combined chlorine to read incorrectly high.
External links
Applications- DuPont Oxone Monopersulfate Compound Applications
- Potassium Monopersulfate – Article on precious metal extraction from distributor Green
Technical