
Phosphine oxide
Encyclopedia
Organophosphorus
Organophosphorus compounds are degradable organic compounds containing carbon–phosphorus bonds , used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment...
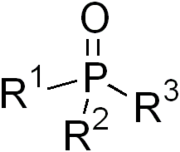
compounds with the formula OPR3, where R = alkyl or aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
. Organophosphine oxides
are considered to be the most stable organophosphorus compounds, triphenylphosphine oxide
Triphenylphosphine oxide
Triphenylphosphine oxide is the chemical compound with the formula OP3. Often chemists abbreviate the formula by writing Ph3PO or PPh3O . This white crystalline compound is a common side product in reactions involving triphenylphosphine...
and trimethylphosphine oxide, decomposing only above 450 °C.
Bonding
The nature of the phosphorus to oxygen "double bond" in phosphine oxides has long been debated. Pentavalent phosphorus is not compatible with the octet ruleOctet rule
The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low (The octet rule is a chemical rule of thumb that states that atoms of low (...
, but phosphorus is known to violate this rule anyway, e.g. phosphorus pentafluoride
Phosphorus pentafluoride
Phosphorus pentafluoride, PF5, is a phosphorus halide. It's a colourless gas at room temperature and pressure.-Structure:Single-crystal X-ray studies indicate PF5 molecule has two distinct P−F bonds : P−Fax = 158.0 pm and P−Feq = 152.2 pm...
and phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
. In older literature the bond is represented as a dative bond, as is currently used to depict an amine oxide
Amine oxide
An amine oxide, also known as amine-N-oxide and N-oxide, is a chemical compound that contains the functional group R3N+-O−, an N-O bond with three additional hydrogen and/or hydrocarbon side chains attached to N. Sometimes it is written as R3N→O or, wrongly, as R3N=O.In the strict sense the...
. The involvement of a phosphorus d-orbitals in bonding is not supported by computational analyses. Alternative theories favor an ionic description
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
R3P+−O−, which explains the short bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
. Molecular Orbital Theory
Molecular orbital theory
In chemistry, molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule...
proposes that the short bond length is attributed to the donation of the lone pair electrons from oxygen to the antibonding phosphorus-carbon bonds. This proposal, which is supported by ab initio calculations, has gained consensus in the chemistry community.
Syntheses
Phosphine oxides are most frequently generated as a by-product of the Wittig reactionWittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
:
- R3PCR'2 + R"2CO → R3PO + R'2C=CR"2
Another common route to phosphine oxides is the thermolysis of phosphonium hydroxides. In the laboratory, phosphine oxides are usually generated by the oxidation, often accidentally, of tertiary phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
s:
- R3P + 1/2 O2 → R3PO
Use
Phosphine oxides are made as by-products in the Wittig reactionWittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
. They themselves are usable in a Wittig-like reaction. So benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
is converted into β-methoxystyrene using methoxymethyl diphenylphosphine oxide in a two step procedure. In step one the phosphine oxide is deprotonated at −90oC in THF/ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
with lithium diisopropylamide
Lithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
, then the aldehyde is added. After aqueous workup, adducts are isolated. With potasium-t-butoxide the adducts are, at room temperature, converted to the styrenes. As the adducts exist as a separatable mixture of D/L compounds and a meso-form the final styrenes are obtainable as pure E- or Z-form .
Parent compound
The parent compound phosphine oxide (H3PO) is unstable. It has been detected with mass spectrometryMass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
as a reaction product of oxygen and phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
, by means of FT-IR in a phosphine - ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
reaction and in matrix isolation
Matrix Isolation
Matrix isolation is an experimental technique used in chemistry and physics which generally involves a material being trapped within an unreactive matrix. A host matrix is a continuous solid phase in which guest particles are embedded. The guest is said to be isolated within the host matrix...
with a reaction of phosphine, vanadium oxytrichloride
Vanadium oxytrichloride
Vanadium oxytrichloride is the inorganic compound with the formula VOCl3. This distillable liquid hydrolyzes readily in air and is a strong oxidant. It is used as a reagent in organic synthesis.-Properties:...
and chromyl chloride
Chromyl chloride
Chromyl chloride is a chemical compound with the formula CrO2Cl2. This compound is an opaque dark blood-red liquid at room temperature and pressure. It is tetrahedral, somewhat like SO2Cl2. CrO2Cl2 is similar to the most commonly encountered chromium derivative chromate, [CrO4]2−; both are...
.It is also been reported relatively stable in a water/ethanol solution by electrochemical oxidation of white phosphorus where is slowly disproportionates into phosphine and hypophosphorous acid
Hypophosphorous acid
Hypophosphorous acid is a phosphorus oxoacid and a powerful reducing agent with molecular formula H3PO2. Inorganic chemists refer to the free acid by this name , or the acceptable name of phosphinic acid. It is a colorless low-melting compound, which is soluble in water, dioxane, and alcohols...
. Phosphine oxide is tautomeric with phosphinous acid (H2POH).
Phosphine oxide is reported as a intermediate in the room-temperature polymerization of phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
and nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
to solid PxHy

