Phosphoryl chloride
Encyclopedia
Phosphoryl chloride is a colourless liquid with the formula 3. It hydrolyses in moist air to phosphoric acid
to release choking fumes of hydrogen chloride
. It is manufactured industrially on a large scale from phosphorus trichloride
and oxygen
or phosphorus pentoxide
. It is mainly used to make phosphate esters such as tricresyl phosphate
.
of 533.5 kJ/mol. On the basis of bond length and electronegativity, the Schomaker-Stevenson rule suggests that the double bond form is very dominant (in contrast with POF3). The P=O bond does not resemble the π bond
in a carbonyl
group as in a ketone. The appropriate description of the P-O interaction is a matter of long discussion. Older textbooks favor a description that invokes participation of the d-orbitals on phosphorus. Some of these d-orbitals project toward the O atom, overlapping with p-orbitals on oxygen. More modern texts seem to favor a description where the P-O π bond
ing involves the σ* components of the P-Cl bonds. These descriptions do not consider a role for d-orbitals.
s to give hydrogen chloride
and phosphoric acid
or phosphate esters, respectively:
If the water is replaced by an alcohol
, the trialkyl phosphate esters result. Such reactions are often performed in the presence of an HCl acceptor such as pyridine
or an amine
. If POCl3 is heated with an excess of a phenol
(Ar
OH) in the presence of a Lewis acid
catalyst such as magnesium chloride
, a triaryl phosphate ester such as triphenyl phosphate
is formed. For example:
POCl3 can also act as a Lewis base, forming adducts with a variety of Lewis acids such as titanium tetrachloride
:
The aluminium chloride
adduct (POCl3·AlCl3) is quite stable, and so POCl3 can be used to remove AlCl3 completely from reaction mixtures at the end of a Friedel-Crafts reaction
. POCl3 reacts with hydrogen bromide
in the presence of AlCl3 to produce POBr3.
with oxygen
at 20-50 °C (air is ineffective):
An alternative synthesis involves the reaction of phosphorus pentachloride and phosphorus pentoxide
. Since these compounds are both solids, a convenient way of performing the reaction is to chlorinate
a mixture of PCl3 and P4O10, which generates the PCl5 in situ. As the PCl3 is consumed, the POCl3 becomes the reaction solvent.
Phosphorus pentachloride also forms POCl3 by reaction with water, but this reaction is less easily controlled than the above reaction.
and tricresyl phosphate
. These esters have been used for many years as flame retardant
s and plasticisers for PVC
. Meanwhile trialkyl esters such as tributyl phosphate
(made similarly from butan-1-ol) are used as liquid-liquid extraction
solvents in nuclear reprocessing
and elsewhere.
In the semiconductor industry, POCl3 is used as a safe liquid phosphorus source in diffusion processes. The phosphorus acts as a dopant used to create N-type layers on a silicon wafer.
In the laboratory, POCl3 is widely used as a dehydrating agent, for example the conversion of amide
s to nitrile
s. Similarly, certain aryl amides can be cyclised to dihydroisoquinoline
derivatives using the Bischler-Napieralski reaction
.
Such reactions are believed to go via an imidoyl chloride; in certain cases where it is stable, the imidoyl chloride is the final product. For example pyridones and pyrimidones can be converted to chloro- derivatives of pyridine
s and pyrimidine
s, which are important intermediates in the pharmaceutical industry.
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
to release choking fumes of hydrogen chloride
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
. It is manufactured industrially on a large scale from phosphorus trichloride
Phosphorus trichloride
Phosphorus trichloride is a chemical compound of phosphorus and chlorine, having chemical formula PCl3. Its shape is trigonal pyramidal. It is the most important of the three phosphorus chlorides. It is an important industrial chemical, being used for the manufacture of organophosphorus compounds...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
or phosphorus pentoxide
Phosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula P4O10 . This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant.-Structure:...
. It is mainly used to make phosphate esters such as tricresyl phosphate
Tricresyl phosphate
Tricresyl phosphate, abbreviated TCP, is an organophosphate compound that is used as a plasticizer and diverse other applications. It is a colourless, viscous liquid, although commercial samples are typically yellow...
.
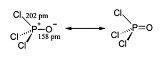
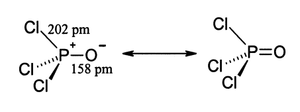
Structure
Like phosphate, phosphoryl chloride is tetrahedral in shape. It features three P-Cl bonds and one very strong P=O double bond, with an estimated bond dissociation energyBond dissociation energy
In chemistry, bond-dissociation energy or D0, is one measure of the bond strength in a chemical bond. It is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K...
of 533.5 kJ/mol. On the basis of bond length and electronegativity, the Schomaker-Stevenson rule suggests that the double bond form is very dominant (in contrast with POF3). The P=O bond does not resemble the π bond
Pi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
in a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group as in a ketone. The appropriate description of the P-O interaction is a matter of long discussion. Older textbooks favor a description that invokes participation of the d-orbitals on phosphorus. Some of these d-orbitals project toward the O atom, overlapping with p-orbitals on oxygen. More modern texts seem to favor a description where the P-O π bond
Pi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
ing involves the σ* components of the P-Cl bonds. These descriptions do not consider a role for d-orbitals.

-
-
- where pm = picometers
-
Chemical properties
POCl3 reacts with water and alcoholAlcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s to give hydrogen chloride
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
and phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
or phosphate esters, respectively:
- O=PCl3 + 3 H2O → O=P(OH)3 + 3 HCl
If the water is replaced by an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
, the trialkyl phosphate esters result. Such reactions are often performed in the presence of an HCl acceptor such as pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
or an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
. If POCl3 is heated with an excess of a phenol
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group bonded directly to an aromatic hydrocarbon group...
(Ar
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
OH) in the presence of a Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
catalyst such as magnesium chloride
Magnesium chloride
Magnesium chloride is the name for the chemical compounds with the formulas MgCl2 and its various hydrates MgCl2x. These salts are typical ionic halides, being highly soluble in water. The hydrated magnesium chloride can be extracted from brine or sea water...
, a triaryl phosphate ester such as triphenyl phosphate
Triphenyl phosphate
Triphenyl phosphate is the chemical compound with the formula OP3. This colourless solid is the ester of phosphoric acid and phenol. It is used as a plasticizer and a fire retardant....
is formed. For example:
- 3 C6H5OH + O=PCl3 → O=P(OC6H5)3 + 3 HCl
POCl3 can also act as a Lewis base, forming adducts with a variety of Lewis acids such as titanium tetrachloride
Titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is an unusual example of a metal halide that is highly volatile...
:
- Cl3P+-O− + TiCl4 → Cl3P+-O−-TiCl4
The aluminium chloride
Aluminium chloride
Aluminium chloride is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large...
adduct (POCl3·AlCl3) is quite stable, and so POCl3 can be used to remove AlCl3 completely from reaction mixtures at the end of a Friedel-Crafts reaction
Friedel-Crafts reaction
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877. There are two main types of Friedel–Crafts reactions: alkylation reactions and acylation reactions. This reaction type is a form of electrophilic aromatic substitution...
. POCl3 reacts with hydrogen bromide
Hydrogen bromide
Hydrogen bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation. Hydrogen bromide and...
in the presence of AlCl3 to produce POBr3.
Preparation
Phosphoryl chloride can be prepared by the reaction of phosphorus trichloridePhosphorus trichloride
Phosphorus trichloride is a chemical compound of phosphorus and chlorine, having chemical formula PCl3. Its shape is trigonal pyramidal. It is the most important of the three phosphorus chlorides. It is an important industrial chemical, being used for the manufacture of organophosphorus compounds...
with oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
at 20-50 °C (air is ineffective):
- 2 PCl3 + O2 → 2 O=PCl3
An alternative synthesis involves the reaction of phosphorus pentachloride and phosphorus pentoxide
Phosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula P4O10 . This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant.-Structure:...
. Since these compounds are both solids, a convenient way of performing the reaction is to chlorinate
Chlorination
Chlorination is the process of adding the element chlorine to water as a method of water purification to make it fit for human consumption as drinking water...
a mixture of PCl3 and P4O10, which generates the PCl5 in situ. As the PCl3 is consumed, the POCl3 becomes the reaction solvent.
- 6 PCl3 + 6 Cl2 → 6 PCl5
- 6 PCl5 + P4O10 → 10 POCl3
Phosphorus pentachloride also forms POCl3 by reaction with water, but this reaction is less easily controlled than the above reaction.
Uses
The most important use for phosphoryl chloride is in the manufacture of triarylphosphate esters (as described above) such as triphenyl phosphateTriphenyl phosphate
Triphenyl phosphate is the chemical compound with the formula OP3. This colourless solid is the ester of phosphoric acid and phenol. It is used as a plasticizer and a fire retardant....
and tricresyl phosphate
Tricresyl phosphate
Tricresyl phosphate, abbreviated TCP, is an organophosphate compound that is used as a plasticizer and diverse other applications. It is a colourless, viscous liquid, although commercial samples are typically yellow...
. These esters have been used for many years as flame retardant
Flame retardant
Flame retardants are chemicals used in thermoplastics, thermosets, textiles and coatings that inhibit or resist the spread of fire. These can be separated into several different classes of chemicals:...
s and plasticisers for PVC
Polyvinyl chloride
Polyvinyl chloride, commonly abbreviated PVC, is a thermoplastic polymer. It is a vinyl polymer constructed of repeating vinyl groups having one hydrogen replaced by chloride. Polyvinyl chloride is the third most widely produced plastic, after polyethylene and polypropylene. PVC is widely used in...
. Meanwhile trialkyl esters such as tributyl phosphate
Tributyl phosphate
Tributyl phosphate, known commonly as TBP, is an organophosphorus compound with the formula 3PO. This colourless, odorless liquid finds some applications as an extractant and a plasticizer. It is an ester of orthophosphoric acid with n-butanol.- Production :Tributyl phosphate is manufactured by...
(made similarly from butan-1-ol) are used as liquid-liquid extraction
Liquid-liquid extraction
Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent. It is an extraction of a substance from one liquid phase into another liquid...
solvents in nuclear reprocessing
Nuclear reprocessing
Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing...
and elsewhere.
In the semiconductor industry, POCl3 is used as a safe liquid phosphorus source in diffusion processes. The phosphorus acts as a dopant used to create N-type layers on a silicon wafer.
In the laboratory, POCl3 is widely used as a dehydrating agent, for example the conversion of amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
s to nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
s. Similarly, certain aryl amides can be cyclised to dihydroisoquinoline
Isoquinoline
Isoquinoline is a heterocyclic aromatic organic compound. It is a structural isomer of quinoline. Isoquinoline and quinoline are benzopyridines, which are composed of a benzene ring fused to a pyridine ring. In a broader sense, the term isoquinoline is used to make reference to isoquinoline...
derivatives using the Bischler-Napieralski reaction
Bischler-Napieralski reaction
The Bischler–Napieralski reaction is an intramolecular electrophilic aromatic substitution reaction that allows for the cyclization of β-arylethylamides or β-arylethylcarbamates. It was first discovered in 1893 by August Bischler and Bernard Napieralski, in affiliation with Basle Chemical Works and...
.
Such reactions are believed to go via an imidoyl chloride; in certain cases where it is stable, the imidoyl chloride is the final product. For example pyridones and pyrimidones can be converted to chloro- derivatives of pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
s and pyrimidine
Pyrimidine
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
s, which are important intermediates in the pharmaceutical industry.
Further reading
- N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
- Handbook of Chemistry and Physics, 71st edition, CRC Press, Ann Arbor, Michigan, 1990.
- J. March, Advanced Organic Chemistry, 4th ed., p. 723, Wiley, New York, 1992.
- The Merck Index, 7th edition, Merck & Co, Rahway, New Jersey, USA, 1960.
- A. D. F. Toy, The Chemistry of Phosphorus, Pergamon Press, Oxford, UK, 1973.
- L. G. Wade, Jr., Organic Chemistry, 6th ed., p. 477, Pearson/Prentice Hall, Upper Saddle River, New Jersey, USA, 2005.
- B. J. Walker, Organophosphorus chemistry, p101-116, Penguin, Harmondsworth, UK, 1972.


