
Ostwald ripening
Encyclopedia
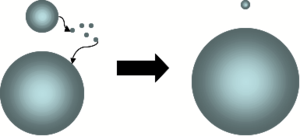
Sol (colloid)
A sol is a colloidal suspension of very small solid particles in a continuous liquid medium. They are quite stable and show the Tyndall effect. Examples include blood, pigmented ink, and paint....
which describes the change of an inhomogeneous structure over time. In other words, over time, small crystals or sol particles dissolve, and redeposit onto larger crystals or sol particles.
Dissolution of small crystals or sol particles and the redeposition of the dissolved species on the surfaces of larger crystals or sol particles was first described by Wilhelm Ostwald
Wilhelm Ostwald
Friedrich Wilhelm Ostwald was a Baltic German chemist. He received the Nobel Prize in Chemistry in 1909 for his work on catalysis, chemical equilibria and reaction velocities...
in 1896. Ostwald ripening is generally found in water-in-oil emulsions, while flocculation
Flocculation
Flocculation, in the field of chemistry, is a process wherein colloids come out of suspension in the form of floc or flakes by the addition of a clarifying agent. The action differs from precipitation in that, prior to flocculation, colloids are merely suspended in a liquid and not actually...
is found in oil-in-water emulsions
Mechanism
This thermodynamicallyThermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
-driven spontaneous process occurs because larger particles are more energetically favored than smaller particles. This stems from the fact that molecules on the surface of a particle are energetically less stable than the ones in the interior. Consider a cubic crystal of atoms: all the atoms inside are bonded to 6 neighbors and are quite stable, but atoms on the surface are only bonded to 5 neighbors or less, which makes these surface atoms less stable. Large particles are more energetically favorable since, continuing with our example, more atoms are bonded to 6 neighbors and fewer atoms are at the unfavorable surface. As the system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
tries to lower its overall energy, molecules on the surface of a small particle (energetically unfavorable, with only 3 or 4 or 5 bonded neighbors) will tend to detach from the particle, as per the Kelvin equation
Kelvin equation
The Kelvin equation describes the change in vapour pressure due to a curved liquid/vapor interface with radius r . The Kelvin equation is used for determination of pore size distribution of a porous medium using adsorption porosimetry...
, and diffuse into the solution. When all small particles do this, it increases the concentration of free atoms in solution. When the free atoms in solution are supersaturated, the free atoms have a tendency to condense on the surface of larger particles.. Therefore, all smaller particles shrink, while larger particles grow, and overall the average size will increase. After an infinite amount of time, the entire population of particles will have become one, huge, spherical particle to minimize the total surface area.
In 1961, Lifshitz and Slyozov performed a mathematical investigation of Ostwald ripening in the case where diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
of material is the slowest process. The derivation first states how a single particle grows in a solution, and this equation describes where the boundary is between small, shrinking particles and large, growing particles. Through a lengthy and abstract mathematical derivation, they conclude that the average radius of the particles
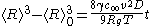
where
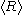 = average radius of all the particles
= average radius of all the particles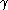 = particle surface tension
= particle surface tensionSurface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
or surface energy
Surface energy
Surface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
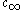 = solubility
= solubilitySolubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
of the particle material
 = molar volume
= molar volumeMolar volume
The molar volume, symbol Vm, is the volume occupied by one mole of a substance at a given temperature and pressure. It is equal to the molar mass divided by the mass density...
of the particle material
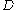 = diffusion coefficient of the particle material
= diffusion coefficient of the particle material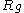 = ideal gas constant
= ideal gas constant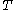 = absolute temperature
= absolute temperatureand
 = time
= timeTwo things should be noted about this growth law: (a) The quantity
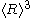 is different from
is different from 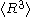 , and only the latter one can be used to calculate average volume, and (b) the statement that
, and only the latter one can be used to calculate average volume, and (b) the statement that 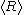 goes as
goes as 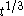 relies on
relies on 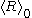 being zero; but because nucleation
being zero; but because nucleationNucleation
Nucleation is the extremely localized budding of a distinct thermodynamic phase. Some examples of phases that may form by way of nucleation in liquids are gaseous bubbles, crystals or glassy regions. Creation of liquid droplets in saturated vapor is also characterized by nucleation...
is a separate process from growth, this places
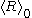 outside the bounds of validity of the equation. In contexts where the actual value of
outside the bounds of validity of the equation. In contexts where the actual value of 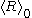 is irrelevant, an approach that respects the meanings of all terms is to take the time derivative of the equation to eliminate
is irrelevant, an approach that respects the meanings of all terms is to take the time derivative of the equation to eliminate 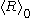 and
and  . Another such approach is to change the
. Another such approach is to change the 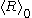 to
to 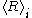 with the initial time
with the initial time 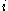 having a positive value.
having a positive value.Also contained in the Lifshitz and Slyozov derivation is an equation for the size distribution function
Distribution function
In molecular kinetic theory in physics, a particle's distribution function is a function of seven variables, f, which gives the number of particles per unit volume in phase space. It is the number of particles per unit volume having approximately the velocity near the place and time...
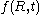 of particles. For convenience, the radius of particles is divided by the average radius to form a new variable,
of particles. For convenience, the radius of particles is divided by the average radius to form a new variable, 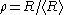 :
: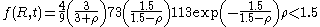
Ironically, at the same time as Lifshitz and Slyozov published their findings, Carl Wagner performed his own mathematical investigation of Ostwald ripening , examining both systems where diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
was slow and also where attachment and detachment at the particle surface was slow. Although his calculations and approach were different, Wagner made exactly the same conclusions as Lifshitz and Slyozov for slow-diffusion systems. This duplicate derivation went unnoticed for years because the two scientific papers were published on opposite sides of the Iron Curtain
Iron Curtain
The concept of the Iron Curtain symbolized the ideological fighting and physical boundary dividing Europe into two separate areas from the end of World War II in 1945 until the end of the Cold War in 1989...
in 1961. It was not until 1975 that Kahlweit addressed the fact that the theories were identical and combined them into the Lifshitz-Slyozov-Wagner or LSW Theory of Ostwald ripening. Many experiments and simulations have proven LSW theory to be robust and accurate. Even some systems that undergo spinodal decomposition
Spinodal decomposition
Spinodal decomposition is a mechanism by which a solution of two or more components can separate into distinct regions with distinctly different chemical compositions and physical properties...
have been shown to quantitatively obey LSW theory after initial stages of growth .
For the curious, Wagner derived that when attachment and detachment of molecules is slower than diffusion, then the growth rate becomes
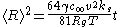
where
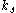 is the reaction rate constant of attachment with units
is the reaction rate constant of attachment with unitsUnits of measurement
A unit of measurement is a definite magnitude of a physical quantity, defined and adopted by convention and/or by law, that is used as a standard for measurement of the same physical quantity. Any other value of the physical quantity can be expressed as a simple multiple of the unit of...
of length per time. Since the average radius is usually something that can be measured in experiments, it is fairly easy to tell if a system is obeying the slow-diffusion equation or the slow-attachment equation. If, of course, the experimental data obeys neither equation, then it is likely that another mechanism is taking place and Ostwald ripening is not occurring.
Although LSW theory and Ostwald ripening were intended for solids ripening in a fluid, Ostwald ripening is also observed in liquid-liquid systems. For example, in an oil-in-water emulsion polymerization
Emulsion polymerization
Emulsion polymerization is a type of radical polymerization that usually starts with an emulsion incorporating water, monomer, and surfactant. The most common type of emulsion polymerization is an oil-in-water emulsion, in which droplets of monomer are emulsified in a continuous phase of water...
, In this case, Ostwald ripening causes the diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
of monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
s (i.e. individual molecules or atoms) from smaller droplets to larger droplets due to greater solubility of the single monomer molecules in the larger monomer droplets. The rate of this diffusion process is linked to the solubility of the monomer in the continuous (water) phase of the emulsion. This can lead to the destabilization of emulsions (for example, by creaming and sedimentation).
Specific examples

Another gastronomical example is in the ouzo effect
Ouzo effect
The ouzo effect is a phenomenon observed when water is added to ouzo and other anise-flavored liqueurs and spirits, such as pastis, raki, arak and absinthe, forming a milky oil-in-water microemulsion...
, where the droplets in the cloudy microemulsion grow by Ostwald ripening.
In geology
Geology
Geology is the science comprising the study of solid Earth, the rocks of which it is composed, and the processes by which it evolves. Geology gives insight into the history of the Earth, as it provides the primary evidence for plate tectonics, the evolutionary history of life, and past climates...
, it is the textural coarsening, aging or growth of phenocrysts and crystals in solid rock which is below the solidus
Solidus (chemistry)
In chemistry, materials science, and physics, the solidus is the locus of temperatures below which a given substance is completely solid...
temperature. It is often ascribed as a process in the formation of orthoclase
Orthoclase
Orthoclase is an important tectosilicate mineral which forms igneous rock. The name is from the Greek for "straight fracture," because its two cleavage planes are at right angles to each other. Alternate names are alkali feldspar and potassium feldspar...
megacryst
Megacryst
In geology, a megacryst is a crystal or grain that is considerably larger than the encircling matrix. They are found in igneous and metamorphic rocks.- Notes :*...
s, as an alternative to the physical processes governing crystal growth from nucleation
Nucleation
Nucleation is the extremely localized budding of a distinct thermodynamic phase. Some examples of phases that may form by way of nucleation in liquids are gaseous bubbles, crystals or glassy regions. Creation of liquid droplets in saturated vapor is also characterized by nucleation...
and growth rate thermochemical
Thermochemistry
Thermochemistry is the study of the energy and heat associated with chemical reactions and/or physical transformations. A reaction may release or absorb energy, and a phase change may do the same, such as in melting and boiling. Thermochemistry focuses on these energy changes, particularly on the...
limitations.
In chemistry, the term refers to the growth of larger crystals from those of smaller size which have a higher solubility than the larger ones. In the process, many small crystals formed initially slowly disappear, except for a few that grow larger, at the expense of the small crystals. The smaller crystals act as fuel for the growth of bigger crystals. Limiting Ostwald ripening is fundamental in modern technology for the solution synthesis of quantum dot
Quantum dot
A quantum dot is a portion of matter whose excitons are confined in all three spatial dimensions. Consequently, such materials have electronic properties intermediate between those of bulk semiconductors and those of discrete molecules. They were discovered at the beginning of the 1980s by Alexei...
s. Ostwald ripening is also the key process in the digestion of precipitates, an important step in gravimetric analysis
Gravimetric analysis
Gravimetric analysis describes a set of methods in analytical chemistry for the quantitative determination of an analyte based on the mass of a solid...
. The digested precipitate is generally purer, and easier to wash and filter.
Ostwald ripening can also occur in emulsion
Emulsion
An emulsion is a mixture of two or more liquids that are normally immiscible . Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsion is used when both the dispersed and the...
systems, with molecules diffusing from small droplets to large ones through the continuous phase. When a miniemulsion
Miniemulsion
A miniemulsion is a special case of emulsion. A miniemulsion is obtained by shearing a mixture comprising two immiscible liquid phases, one surfactant and one co-surfactant ....
is desired, an extremely hydrophobic compound is added to stop this process from taking place.
See also
- Rock microstructureRock microstructureRock microstructure includes the texture of a rock and the small scale rock structures. The words "texture" and "microstructure" are interchangeable, with the latter preferred in modern geological literature...
- Kelvin equationKelvin equationThe Kelvin equation describes the change in vapour pressure due to a curved liquid/vapor interface with radius r . The Kelvin equation is used for determination of pore size distribution of a porous medium using adsorption porosimetry...
- Kirkendall effectKirkendall effectThe Kirkendall effect is the motion of the boundary layer between two metals that occurs as a consequence of the difference in diffusion rates of the metal atoms...
- Critical radiusCritical radiusCritical radius is the minimum size that must be formed by atoms or molecules clustering together before a new-phase inclusion is stable and begins to grow. Formation of such stable "nuclei" is called nucleation. In precipitation models this is generally a prelude to models of the growth...
- Solubility equilibrium#Particle size effect
- FlocculationFlocculationFlocculation, in the field of chemistry, is a process wherein colloids come out of suspension in the form of floc or flakes by the addition of a clarifying agent. The action differs from precipitation in that, prior to flocculation, colloids are merely suspended in a liquid and not actually...
- AggregationParticle aggregationParticle aggregation in materials science is direct mutual attraction between particles via van der Waals forces or chemical bonding....
- CoalescenceCoalescenceCoalescence may refer to:* Coalescence , the merging of genetic lineages backwards time to a most recent common ancestor* Coalescence , the merging of two or more phonological segments into one...
External links
- Ostwald Ripening a 3D Kinetic Monte Carlo simulation

