
Critical radius
Encyclopedia
Critical radius is the minimum size that must be formed by atoms or molecules clustering together (in a gas, liquid or solid matrix) before a new-phase inclusion (a bubble, a droplet, or a solid particle) is stable and begins to grow. Formation of such stable "nuclei" is called nucleation
. In precipitation models this is generally a prelude to models of the growth process itself. Sometimes precipitation is rate-limited by the nucleation process. This happens for example before one takes a cup of superheated water from a microwave and, when jiggling it against dust particles on the wall of the cup, enables "heterogeneous" nucleation that then rapidly converts much of that water into steam.
If the change in phase forms a crystalline solid in a liquid matrix, the atoms might then form a dendrite
. The crystal
growth continues in three dimensions, the atoms attaching themselves in certain preferred directions, usually along the axes of a crystal, forming a characteristic tree-like structure of a dendrite.
example: the critical radius for spheric-like dendride in an ideal system is
Gibs free energy
G=-4/3π r3 Gv+ 4π r2γ
Gv is Gibs volume energy and γ is the interfacial energy. The critical radius is found by setting the derivative of G equal to zero
dg/dr=-4π rc2 Gv+ 8π rcγ = 0
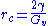 ,
,
where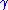 is the surface energy, and
is the surface energy, and  is Gibbs energy per volume.
is Gibbs energy per volume.
Nucleation
Nucleation is the extremely localized budding of a distinct thermodynamic phase. Some examples of phases that may form by way of nucleation in liquids are gaseous bubbles, crystals or glassy regions. Creation of liquid droplets in saturated vapor is also characterized by nucleation...
. In precipitation models this is generally a prelude to models of the growth process itself. Sometimes precipitation is rate-limited by the nucleation process. This happens for example before one takes a cup of superheated water from a microwave and, when jiggling it against dust particles on the wall of the cup, enables "heterogeneous" nucleation that then rapidly converts much of that water into steam.
If the change in phase forms a crystalline solid in a liquid matrix, the atoms might then form a dendrite
Dendrite (crystal)
A crystal dendrite is a crystal that develops with a typical multi-branching tree-like form. Dendritic crystal growth is very common and illustrated by snowflake formation and frost patterns on a window. Dendritic crystallization forms a natural fractal pattern...
. The crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
growth continues in three dimensions, the atoms attaching themselves in certain preferred directions, usually along the axes of a crystal, forming a characteristic tree-like structure of a dendrite.
example: the critical radius for spheric-like dendride in an ideal system is
Gibs free energy
G=-4/3π r3 Gv+ 4π r2γ
Gv is Gibs volume energy and γ is the interfacial energy. The critical radius is found by setting the derivative of G equal to zero
dg/dr=-4π rc2 Gv+ 8π rcγ = 0
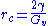 ,
,where
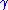 is the surface energy, and
is the surface energy, and  is Gibbs energy per volume.
is Gibbs energy per volume.

