
Opsin
Encyclopedia
Atomic mass unit
The unified atomic mass unit or dalton is a unit that is used for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state, and has a value of...
membrane-bound G protein-coupled receptor
G protein-coupled receptor
G protein-coupled receptors , also known as seven-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptor, and G protein-linked receptors , comprise a large protein family of transmembrane receptors that sense molecules outside the cell and activate inside signal...
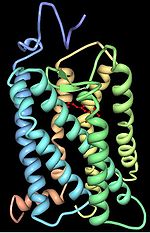
s of the retinylidene protein
Retinylidene protein
Retinylidene proteins are a family of proteins that use retinal as chromophore for light reception. Proteins of this family are also called opsins...
family found in photoreceptor cells of the retina
Retina
The vertebrate retina is a light-sensitive tissue lining the inner surface of the eye. The optics of the eye create an image of the visual world on the retina, which serves much the same function as the film in a camera. Light striking the retina initiates a cascade of chemical and electrical...
. Five classical groups of opsins are involved in vision, mediating the conversion of a photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
of light into an electrochemical signal, the first step in the visual transduction cascade. Another opsin found in the mammalian retina, melanopsin
Melanopsin
Melanopsin is a photopigment found in specialized photosensitive ganglion cells of the retina that are involved in the regulation of circadian rhythms, pupillary light reflex, and other non-visual responses to light. In structure, melanopsin is an opsin, a retinylidene protein variety of...
, is involved in circadian rhythm
Circadian rhythm
A circadian rhythm, popularly referred to as body clock, is an endogenously driven , roughly 24-hour cycle in biochemical, physiological, or behavioural processes. Circadian rhythms have been widely observed in plants, animals, fungi and cyanobacteria...
s and pupillary reflex but not in image-forming.
Opsin classification
There are two groups of protein termed opsins; these groups are not homologous, but have convergently evolved a similar form and function. Type I opsins are employed by prokaryotes, whereas animals use type II opsins. No opsins have been found outside these groups (for instance in plants, fungi, or placozoans), although as-yet unconfirmed reports of opsins in sponges would suggest that opsins were present in the ancestral metazoan.Based on the phylogeny of their sequences, type 2 opsins can be grouped into six families; these families are very distinct, with under 20% of their sequences shared with any other subfamily. The families consist of the vertebrate opsins/encephalopsins; Go opsins; Gs opsins; invertebrate Gq opsins; the photoisomerases and neuropsins. These subfamilies can be grouped according to their expression; the first three are found in ciliary-type photoreceptor cells; Gq opsins in rhabdomeric-type photoreceptor cells; and the latter two are found elsewhere but based on their shared intron positions can be bundled together into the photoisomerases.
Prokaryotic (type 1) opsins
Like eukaryotic opsins, prokaryotic opsins have a seven transmembrane domain structure similar to that found in eukaryotic G-protein coupled receptors. Despite this similarity, there is no evidence that they are evolutionarily related, suggesting that they evolved independently of one another.Several type 1 opsins, such as proteo-
Proteorhodopsin
Proteorhodopsin is a photoactive retinylidene protein in marine bacterioplanktons and eukaryotes. Just like the homologous pigment bacteriorhodopsin found in some archaea, it consists of a transmembrane protein bound to a retinal molecule and functions as a light-driven proton pump. Some members...
, halo- and bacteriorhodopsin
Bacteriorhodopsin
Bacteriorhodopsin is a protein used by Archaea, the most notable one being Halobacteria. It acts as a proton pump; that is, it captures light energy and uses it to move protons across the membrane out of the cell...
, are used by various bacterial groups to harvest energy from light to carry out metabolic processes using a non-chlorophyll
Chlorophyll
Chlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
-based pathway. Additionally, sensory rhodopsins exist in Halobacteria
Halobacteria
In taxonomy, the Halobacteria are a class of the Euryarchaeota, found in water saturated or nearly saturated with salt. They are also called halophiles, though this name is also used for other organisms which live in somewhat less concentrated salt water...
that induce a phototactic
Phototaxis
Phototaxis is a kind of taxis, or locomotory movement, that occurs when a whole organism moves in response to the stimulus of light. This is advantageous for phototrophic organisms as they can orient themselves most efficiently to receive light for photosynthesis...
response by interacting with transducer
Signal transduction
Signal transduction occurs when an extracellular signaling molecule activates a cell surface receptor. In turn, this receptor alters intracellular molecules creating a response...
membrane-embedded proteins that have no relation to G proteins.
Ciliary opsins
Ciliary opsins are expressed in ciliary photoreceptor cells, and include the vertebrate opsins/encephalopsins, Go and Gs opsin subfamilies. They convert light signals to nerve impulses via cyclic nucleotide gated ion channels, which work by increasing the charge differential across the cell membrane (i.e. hyperpolarizationHyperpolarization (biology)
Hyperpolarization is a change in a cell's membrane potential that makes it more negative. It is the opposite of a depolarization.Hyperpolarization is often caused by efflux of K+ through K+ channels, or influx of Cl– through Cl– channels. On the other hand, influx of cations, e.g...
.)
Vertebrate opsins
Vertebrate opsins can be further subdivided into rod opsins and four types of cone opsin, based on differential spatial expression, spectral sensitivity, and evolutionEvolution
Evolution is any change across successive generations in the heritable characteristics of biological populations. Evolutionary processes give rise to diversity at every level of biological organisation, including species, individual organisms and molecules such as DNA and proteins.Life on Earth...
ary history. Rod opsins (rhodopsins, usually denoted Rh), are used in night vision, are thermally stable, and are found in the rod photoreceptor cells
Rod cell
Rod cells, or rods, are photoreceptor cells in the retina of the eye that can function in less intense light than can the other type of visual photoreceptor, cone cells. Named for their cylindrical shape, rods are concentrated at the outer edges of the retina and are used in peripheral vision. On...
. Cone opsins, employed in color vision, are less-stable opsins located in the cone photoreceptor cells
Cone cell
Cone cells, or cones, are photoreceptor cells in the retina of the eye that are responsible for color vision; they function best in relatively bright light, as opposed to rod cells that work better in dim light. If the retina is exposed to an intense visual stimulus, a negative afterimage will be...
. Cone opsins are further subdivided according to their absorption maxima (λmax), the wavelength at which the highest light absorption is observed. Evolutionary relationships, deduced using the amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
sequence of the opsins, are also frequently used to categorize cone opsins into their respective group. Both methods predict four general cone opsin groups in addition to rhodopsin.
Humans have the following set of photoreceptor proteins responsible for vision:
- RhodopsinRhodopsinRhodopsin, also known as visual purple, is a biological pigment of the retina that is responsible for both the formation of the photoreceptor cells and the first events in the perception of light. Rhodopsins belong to the G-protein coupled receptor family and are extremely sensitive to light,...
(Rh1, OPN2, RHORhoRho is the 17th letter of the Greek alphabet. In the system of Greek numerals, it has a value of 100. It is derived from Semitic resh "head"...
) – expressed in rod cellRod cellRod cells, or rods, are photoreceptor cells in the retina of the eye that can function in less intense light than can the other type of visual photoreceptor, cone cells. Named for their cylindrical shape, rods are concentrated at the outer edges of the retina and are used in peripheral vision. On...
s, used in night vision - Three cone opsins (also known as photopsinPhotopsinPhotopsins are the photoreceptor proteins found in the cone cells of the retina that are the basis of color vision. Photopsins are very close analogs of the visual purple rhodopsin that is used in night vision...
s) – expressed in cone cellCone cellCone cells, or cones, are photoreceptor cells in the retina of the eye that are responsible for color vision; they function best in relatively bright light, as opposed to rod cells that work better in dim light. If the retina is exposed to an intense visual stimulus, a negative afterimage will be...
s, used in color visionColor visionColor vision is the capacity of an organism or machine to distinguish objects based on the wavelengths of the light they reflect, emit, or transmit...
- Long Wavelength Sensitive (OPN1LWOPN1LWRed-sensitive opsin is a protein that in humans is encoded by the OPN1LW gene.-External links:* -Further reading:...
) Opsin – λmax in the red region of the electromagnetic spectrum - Middle Wavelength Sensitive (OPN1MWOPN1MWGreen-sensitive opsin is a protein that in humans is encoded by the OPN1MW gene.-External links:* -Further reading:...
) Opsin – λmax in the green region of the electromagnetic spectrum - Short Wavelength Sensitive (OPN1SWOPN1SWBlue-sensitive opsin is a protein that in humans is encoded by the OPN1SW gene.-Further reading:...
) Opsin – λmax in the blue region of the electromagnetic spectrum
- Long Wavelength Sensitive (OPN1LW
Encephalopsins
This type of opsin is expressed throughout the mammalian body.It is also expressed in ciliary photoreceptor cells in annelids, and in the brains of some insects.
Go / Gs opsins
These opsins, absent from higher vertebrates and arthropods, are found in the ciliary photoreceptor cells of molluscs and basal chordates (amphioxus); and in cnidarians, respectively.Rhabdomeric opsins
Arthropods and molluscs use Gq opsins. Arthropods appear to attain colour vision in a similar fashion to the vertebrates, by the use of three (or more) distinct groups of opsin, distinct both in terms of phylogeny and spectral sensitivity. The Gq opsin melanopsin is also expressed in vertebrates, where it is responsible for the maintenance of circadian rhythmCircadian rhythm
A circadian rhythm, popularly referred to as body clock, is an endogenously driven , roughly 24-hour cycle in biochemical, physiological, or behavioural processes. Circadian rhythms have been widely observed in plants, animals, fungi and cyanobacteria...
s.
Unlike ciliary opsins, these are associated with canonical transient receptor potential ion channels; these lead to the electric potential difference across a cell membrane being eradicated (i.e. depolarization).
The identification of the crystal structure of squid rhodopsin is likely to further our understanding of its function in this group.
Arthropods do use different opsins in their different eye types, but at least in Limulus the opsins expressed in lateral and in compound eyes are 99% identical and presumably diverged recently.
Photoisomerases
This class of opsins are not coupled to a G-protein, and thus serve to traffic retinal around in response to light, rather than directly in signal-induction.Neuropsins
These opsins are found in nervous tissue in mammals, and despite some genetic similarities to photoisomerases, their function has not yet been identified.| Name | Gene | Notes |
|---|---|---|
| Melanopsin Melanopsin Melanopsin is a photopigment found in specialized photosensitive ganglion cells of the retina that are involved in the regulation of circadian rhythms, pupillary light reflex, and other non-visual responses to light. In structure, melanopsin is an opsin, a retinylidene protein variety of... |
OPN4 | best studied novel opsin involved in circadian rhythms and pupillary reflex |
| Pineal Opsin (Pinopsin) | wide range of expression in the brain, most notably in the pineal region Pineal gland The pineal gland is a small endocrine gland in the vertebrate brain. It produces the serotonin derivative melatonin, a hormone that affects the modulation of wake/sleep patterns and seasonal functions... |
|
| Vertebrate Ancient (VA) opsin | has three isoforms VA short (VAS), VA medium (VAM), and VA long (VAL). It is expressed in the inner retina, within the horizontal and amacrine cells, as well as the pineal organ and habenular region of the brain | |
| Parapinopsin (PP) Opsin | ||
| Extraretinal (or extra-ocular) Rhodopsin-Like Opsins (Exo-Rh) | Rhodopsin-like protein expressed in the pineal region | |
| Encephalopsin or Panopsin | OPN3 OPN3 Opsin-3 is a protein that, in humans, is encoded by the OPN3 gene. Alternative splicing of this gene results in multiple transcript variants encoding different protein isoforms.- Function :Opsins are members of the G protein-coupled receptor superfamily... |
originally found in human and mice tissue with a very wide range of expression (brain, testes, heart, liver, kidney, skeletal muscle, lung, pancreas and retina) |
| Teleost Multiple Tissue (TMT) Opsin | Teleost fish opsin with a wide range of expression | |
| Peropsin or "Retinal pigment epithelium-derived rhodopsin homolog" | RRH RRH Visual pigment-like receptor peropsin is a protein that in humans is encoded by the RRH gene.-Further reading:... |
expressed in the retinal pigment epithelium (RPE) cells |
| Retinal G protein coupled receptor Retinal G protein coupled receptor RPE-retinal G protein-coupled receptor is a protein that in humans is encoded by the RGR gene.-Interactions:Retinal G protein coupled receptor has been shown to interact with KIAA1279.-Further reading:... |
RGR | expressed in the retinal pigment epithelium (RPE) and Müller cells |
| Neuropsin | OPN5 OPN5 Opsin-5 is a protein that in humans is encoded by the OPN5 gene.-Further reading:... |
|
Structure and function
Opsin proteins covalently bind to a vitamin AVitamin A
Vitamin A is a vitamin that is needed by the retina of the eye in the form of a specific metabolite, the light-absorbing molecule retinal, that is necessary for both low-light and color vision...
-based retinaldehyde chromophore
Chromophore
A chromophore is the part of a molecule responsible for its color. The color arises when a molecule absorbs certain wavelengths of visible light and transmits or reflects others. The chromophore is a region in the molecule where the energy difference between two different molecular orbitals falls...
through a Schiff base linkage to a lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
residue in the seventh transmembrane alpha helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
. In vertebrates, the chromophore is either 11-cis
Cis
Cis may have the following meanings:* "Cis-" as a prefix of Latin origin, meaning "on the same side [as]" or "on this side [of]", with several derived usages:** In chemistry, cis- refers to cis-trans isomerism...
-retinal (A1) or 11-cis-3,4-didehydroretinal (A2) and is found in the retinal binding pocket of the opsin. The absorption of a photon of light results in the photoisomerisation of the chromophore from the 11-cis to an all-trans conformation. The photoisomerization induces a conformational change in the opsin protein, causing the activation of the phototransduction cascade. The opsin remains insensitive to light in the trans form. It is regenerated by the replacement of the all-trans retinal by a newly synthesized 11-cis-retinal provided from the retinal epithelial cells. Opsins are functional while bound to either chromophore, with A2-bound opsin λmax being at a longer wavelength than A1-bound opsin.
Opsins contain seven transmembrane α-helical domains connected by three extra-cellular and three cytoplasm
Cytoplasm
The cytoplasm is a small gel-like substance residing between the cell membrane holding all the cell's internal sub-structures , except for the nucleus. All the contents of the cells of prokaryote organisms are contained within the cytoplasm...
ic loops. Many amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
residues, termed functionally conserved residues, are highly conserved between all opsin groups, indicative of important functional roles. All residue positions discussed henceforth are relative to the 348 amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
bovine rhodopsin crystallized by Palczewski et al.. Lys296 is conserved in all known opsins and serves as the site for the Schiff base linkage with the chromophore. Cys138 and Cys110 form a highly conserved disulfide bridge. Glu113 serves as the counterion, stabilizing the protonation of the Schiff linkage between Lys296 and the chromophore. The Glu134-Arg135-Tyr136 is another highly conserved motif, involved in the propagation of the transduction signal once a photon has been absorbed.
Certain amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
residues, termed spectral tuning sites, have a strong effect on λmax values. Using site-directed mutagenesis
Site-directed mutagenesis
Site-directed mutagenesis, also called site-specific mutagenesis or oligonucleotide-directed mutagenesis, is a molecular biology technique in which a mutation is created at a defined site in a DNA molecule. In general, this form of mutagenesis requires that the wild type gene sequence be known...
, it is possible to selectively mutate these residues and investigate the resulting changes in light absorption properties of the opsin. It is important to differentiate spectral tuning sites, residues that affect the wavelength at which the opsin absorbs light, from functionally conserved sites, residues important for the proper functioning of the opsin. They are not mutually exclusive, but, for practical reasons, it is easier to investigate spectral tuning sites that do not affect opsin functionality. For a comprehensive review of spectral tuning sites see Yokoyama and Deeb. The impact of spectral tuning sites on λmax differs between different opsin groups and between opsin groups of different species.
External links
- Review of opsins and current research:
- Illustration at Baldwin-Wallace CollegeBaldwin-Wallace CollegeBaldwin–Wallace College is a liberal arts college in Berea, Ohio, founded in 1845. It is home to the Riemenschneider-Bach Institute and the Baldwin–Wallace Conservatory of Music, an internationally renowned music school. The college is affiliated with the United Methodist Church. Students receive a...

