
Bacteriorhodopsin
Encyclopedia
Bacteriorhodopsin is a protein used by Archaea
, the most notable one being Halobacteria
. It acts as a proton pump
; that is, it captures light energy and uses it to move proton
s across the membrane out of the cell. The resulting proton gradient is subsequently converted into chemical energy.
Bacteriorhodopsin is an integral membrane protein
usually found in two-dimensional crystalline patches known as "purple membrane", which can occupy up to nearly 50% of the surface area of the archaeal cell. The repeating element of the hexagonal lattice is composed of three identical protein chains, each rotated by 120 degrees relative to the others. Each chain has seven transmembrane alpha helices
and contains one molecule of retinal
buried deep within, the typical structure for retinylidene protein
s.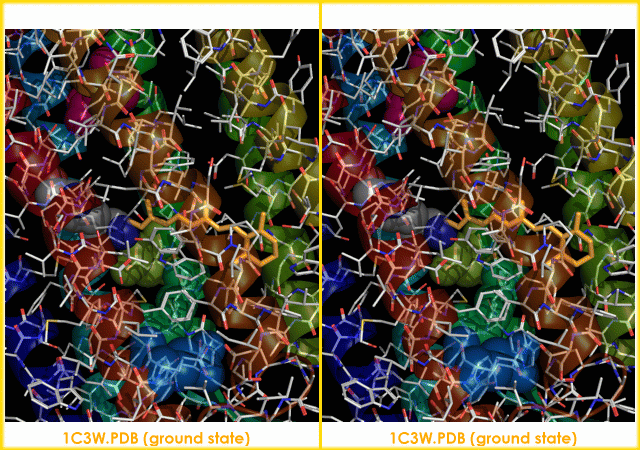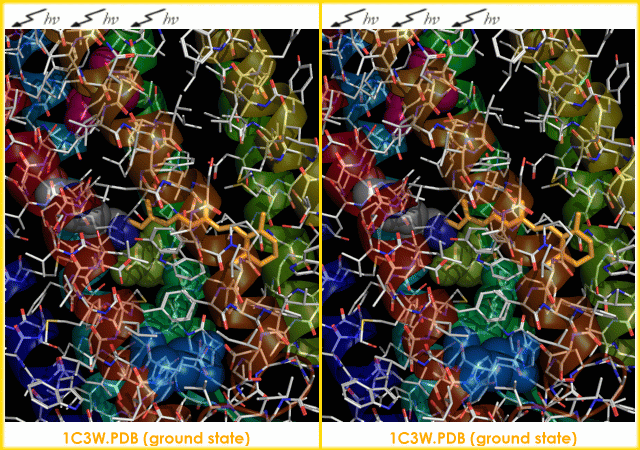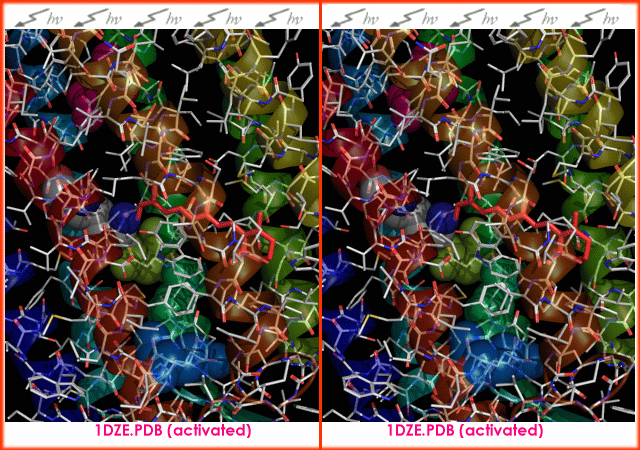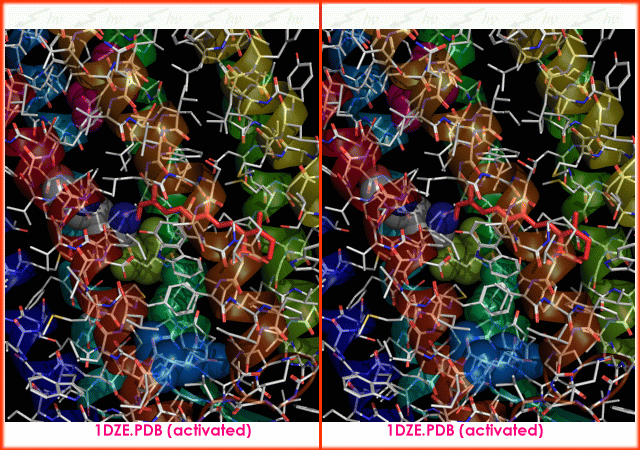 It is the retinal molecule that changes its conformation when absorbing a photon
It is the retinal molecule that changes its conformation when absorbing a photon
, resulting in a conformational change
of the surrounding protein and the proton pumping action. It is covalently linked to Lys216 in the chromophore
by Schiff base
action. After photoisomerization of the retinal molecule, Asp85 becomes a proton acceptor of the donor proton from the retinal molecule. This releases a proton from a "holding site" into the extracellular side (EC) of the membrane. Reprotonation of the retinal molecule by Asp96 restores its original isomerized form. This results in a second proton being released to the EC side. Asp85 releases its proton into the "holding site," where a new cycle may begin.
The bacteriorhodopsin molecule is purple and is most efficient at absorbing green light (wavelength 500-650 nm, with the absorption maximum at 568 nm).
Bacteriorhodopsin belongs to a family of bacterial proteins
related to vertebrate
rhodopsin
s, the pigment
s that sense light in the retina
. Rhodopsins also contain retinal; however, the functions of rhodopsin and bacteriorhodopsin are different, and there is only slight homology
in their amino acid
sequences. Both rhodopsin and bacteriorhodopsin belong to the 7TM receptor family of proteins, but rhodopsin is a G protein-coupled receptor
and bacteriorhodopsin is not. In the first use of electron crystallography
to obtain an atomic-level protein structure
, the structure of bacteriorhodopsin was resolved in 1990. It was then used as a template to build models of G protein-coupled receptors before crystallographic structures
were also available for these protein
s.
Many molecules have homology to bacteriorhodopsin, including the light-driven chloride pump halorhodopsin
(for which the crystal structure is also known), and some directly light-activated channels like channelrhodopsin
.
All other photosynthetic systems in bacteria, algae, and plants use chlorophyll
s or bacteriochlorophyll
s rather than bacteriorhodopsin. These also produce a proton gradient, but in a quite different and more indirect way involving an electron transfer chain consisting of several other proteins. Furthermore, chlorophylls are aided in capturing light energy by other pigments known as "antennas"; these are not present in bacteriorhodopsin-based systems. Last, chlorophyll-based photosynthesis is coupled to carbon fixation
(the incorporation of carbon dioxide
into larger organic molecules); this is not true for bacteriorhodopsin-based system. Thus, it is likely that photosynthesis independently evolved at least twice, once in bacteria and once in archaea.
Archaea
The Archaea are a group of single-celled microorganisms. A single individual or species from this domain is called an archaeon...
, the most notable one being Halobacteria
Halobacteria
In taxonomy, the Halobacteria are a class of the Euryarchaeota, found in water saturated or nearly saturated with salt. They are also called halophiles, though this name is also used for other organisms which live in somewhat less concentrated salt water...
. It acts as a proton pump
Proton pump
A proton pump is an integral membrane protein that is capable of moving protons across a cell membrane, mitochondrion, or other organelle. Mechanisms are based on conformational changes of the protein structure or on the Q cycle.-Function:...
; that is, it captures light energy and uses it to move proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s across the membrane out of the cell. The resulting proton gradient is subsequently converted into chemical energy.
Bacteriorhodopsin is an integral membrane protein
Integral membrane protein
An integral membrane protein is a protein molecule that is permanently attached to the biological membrane. Proteins that cross the membrane are surrounded by "annular" lipids, which are defined as lipids that are in direct contact with a membrane protein...
usually found in two-dimensional crystalline patches known as "purple membrane", which can occupy up to nearly 50% of the surface area of the archaeal cell. The repeating element of the hexagonal lattice is composed of three identical protein chains, each rotated by 120 degrees relative to the others. Each chain has seven transmembrane alpha helices
Transmembrane helix
Transmembrane domain usually denotes a single transmembrane alpha helix of a transmembrane protein. It is called a "domain" because an alpha-helix in a membrane can fold independently from the rest of the protein, similar to domains of water-soluble proteins...
and contains one molecule of retinal
Retinal
Retinal, also called retinaldehyde or vitamin A aldehyde, is one of the many forms of vitamin A . Retinal is a polyene chromophore, and bound to proteins called opsins, is the chemical basis of animal vision...
buried deep within, the typical structure for retinylidene protein
Retinylidene protein
Retinylidene proteins are a family of proteins that use retinal as chromophore for light reception. Proteins of this family are also called opsins...
s.

Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
, resulting in a conformational change
Conformational change
A macromolecule is usually flexible and dynamic. It can change its shape in response to changes in its environment or other factors; each possible shape is called a conformation, and a transition between them is called a conformational change...
of the surrounding protein and the proton pumping action. It is covalently linked to Lys216 in the chromophore
Chromophore
A chromophore is the part of a molecule responsible for its color. The color arises when a molecule absorbs certain wavelengths of visible light and transmits or reflects others. The chromophore is a region in the molecule where the energy difference between two different molecular orbitals falls...
by Schiff base
Schiff base
A Schiff base, named after Hugo Schiff, is a compound with a functional group that contains a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group, not hydrogen....
action. After photoisomerization of the retinal molecule, Asp85 becomes a proton acceptor of the donor proton from the retinal molecule. This releases a proton from a "holding site" into the extracellular side (EC) of the membrane. Reprotonation of the retinal molecule by Asp96 restores its original isomerized form. This results in a second proton being released to the EC side. Asp85 releases its proton into the "holding site," where a new cycle may begin.
The bacteriorhodopsin molecule is purple and is most efficient at absorbing green light (wavelength 500-650 nm, with the absorption maximum at 568 nm).
Bacteriorhodopsin belongs to a family of bacterial proteins
Bacterial rhodopsins
Bacterial rhodopsins are a family of bacterial opsins. They are retinal-binding proteins that provide light-dependent ion transport and sensory functions to a family of halophilic and other bacteria...
related to vertebrate
Vertebrate
Vertebrates are animals that are members of the subphylum Vertebrata . Vertebrates are the largest group of chordates, with currently about 58,000 species described. Vertebrates include the jawless fishes, bony fishes, sharks and rays, amphibians, reptiles, mammals, and birds...
rhodopsin
Rhodopsin
Rhodopsin, also known as visual purple, is a biological pigment of the retina that is responsible for both the formation of the photoreceptor cells and the first events in the perception of light. Rhodopsins belong to the G-protein coupled receptor family and are extremely sensitive to light,...
s, the pigment
Pigment
A pigment is a material that changes the color of reflected or transmitted light as the result of wavelength-selective absorption. This physical process differs from fluorescence, phosphorescence, and other forms of luminescence, in which a material emits light.Many materials selectively absorb...
s that sense light in the retina
Retina
The vertebrate retina is a light-sensitive tissue lining the inner surface of the eye. The optics of the eye create an image of the visual world on the retina, which serves much the same function as the film in a camera. Light striking the retina initiates a cascade of chemical and electrical...
. Rhodopsins also contain retinal; however, the functions of rhodopsin and bacteriorhodopsin are different, and there is only slight homology
Homology (biology)
Homology forms the basis of organization for comparative biology. In 1843, Richard Owen defined homology as "the same organ in different animals under every variety of form and function". Organs as different as a bat's wing, a seal's flipper, a cat's paw and a human hand have a common underlying...
in their amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
sequences. Both rhodopsin and bacteriorhodopsin belong to the 7TM receptor family of proteins, but rhodopsin is a G protein-coupled receptor
G protein-coupled receptor
G protein-coupled receptors , also known as seven-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptor, and G protein-linked receptors , comprise a large protein family of transmembrane receptors that sense molecules outside the cell and activate inside signal...
and bacteriorhodopsin is not. In the first use of electron crystallography
Electron crystallography
Electron crystallography is a method to determine the arrangement of atoms in solids using a transmission electron microscope .- Comparison with X-ray crystallography :...
to obtain an atomic-level protein structure
Protein structure
Proteins are an important class of biological macromolecules present in all organisms. Proteins are polymers of amino acids. Classified by their physical size, proteins are nanoparticles . Each protein polymer – also known as a polypeptide – consists of a sequence formed from 20 possible L-α-amino...
, the structure of bacteriorhodopsin was resolved in 1990. It was then used as a template to build models of G protein-coupled receptors before crystallographic structures
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
were also available for these protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s.
Many molecules have homology to bacteriorhodopsin, including the light-driven chloride pump halorhodopsin
Halorhodopsin
Halorhodopsin is a light-driven ion pump, specific for chloride ions, and found in phylogenetically ancient archaea, known as halobacteria...
(for which the crystal structure is also known), and some directly light-activated channels like channelrhodopsin
Channelrhodopsin
Channelrhodopsins are a subfamily of opsin proteins that function as light-gated ion channels. They serve as sensory photoreceptors in unicellular green algae, controlling phototaxis, i.e. movement in response to light. Expressed in cells of other organisms, they enable the use of light to control...
.
All other photosynthetic systems in bacteria, algae, and plants use chlorophyll
Chlorophyll
Chlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
s or bacteriochlorophyll
Bacteriochlorophyll
Bacteriochlorophylls are photosynthetic pigments that occur in various phototrophic bacteria. They were discovered by Von Neil in 1932 . They are related to chlorophylls, which are the primary pigments in plants, algae, and cyanobacteria. Groups that contain bacteriochlorophyll conduct...
s rather than bacteriorhodopsin. These also produce a proton gradient, but in a quite different and more indirect way involving an electron transfer chain consisting of several other proteins. Furthermore, chlorophylls are aided in capturing light energy by other pigments known as "antennas"; these are not present in bacteriorhodopsin-based systems. Last, chlorophyll-based photosynthesis is coupled to carbon fixation
Carbon fixation
In biology, carbon fixation is the reduction of carbon dioxide to organic compounds by living organisms. The obvious example is photosynthesis. Carbon fixation requires both a source of energy such as sunlight, and an electron donor such as water. All life depends on fixed carbon. Organisms that...
(the incorporation of carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
into larger organic molecules); this is not true for bacteriorhodopsin-based system. Thus, it is likely that photosynthesis independently evolved at least twice, once in bacteria and once in archaea.
See also
- Bacterial rhodopsinsBacterial rhodopsinsBacterial rhodopsins are a family of bacterial opsins. They are retinal-binding proteins that provide light-dependent ion transport and sensory functions to a family of halophilic and other bacteria...
- Protein-coated discProtein-coated discProtein-Coated Disc is a theoretical optical disc technology currently being developed by Professor Venkatesan Renugopalakrishnan, formerly of Harvard Medical School and Florida International University. PCD would greatly increase storage over Holographic Versatile Disc optical disc systems...
- theoretical data storage capacity of 50 terabyteTerabyteThe terabyte is a multiple of the unit byte for digital information. The prefix tera means 1012 in the International System of Units , and therefore 1 terabyte is , or 1 trillion bytes, or 1000 gigabytes. 1 terabyte in binary prefixes is 0.9095 tebibytes, or 931.32 gibibytes...
s

