
Non-Kekulé molecule
Encyclopedia
A non-Kekulé molecule is a conjugated
hydrocarbon
that cannot be assigned classical Kekulé structures. Since non-Kekulé molecules have two or more formal radical
centers, their spin-spin
interactions can cause electrical conductivity or ferromagnetism
(molecule-based magnets), and applications to functional materials are expected. However, as these molecules are quite reactive and most of them are easily decomposed or polymerized
at room temperature, strategies for stabilization are needed for their practical use. Synthesis and observation of these reactive molecules are generally accomplished by matrix-isolation methods.
The simplest non-Kekulé molecules are biradicals.
is an even-electron chemical compound
with two free radical centres which act independently of each other and not to be confused with diradical
s in general.
One of the first biradicals was synthesized by Wilhelm Schlenk
in 1915 following the same methodology as Moses Gomberg
's triphenylmethyl radical
. The so-called Schlenk-Brauns hydrocarbons are:
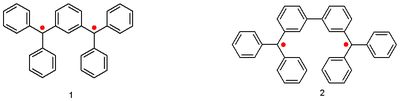 Eugene Müller, with the aid of a Gouy balance, established for the first time that these compounds are paramagnetic with a triplet ground state
Eugene Müller, with the aid of a Gouy balance, established for the first time that these compounds are paramagnetic with a triplet ground state
.
Other classic biradicals are those synthesed by Tschitschibabin , Yang and Coppinger .
 This molecule can be obtained from photolysis of a diazo
This molecule can be obtained from photolysis of a diazo
precursor with expulsion of nitrogen or from photolysis of 2-methylenecyclobutanone with expulsion of carbon monoxide. In 1966 Paul Dowd determined with electron spin resonance that this compound also has a triplet state
. In a crystalline host the 6 hydrogen atoms in TMM are identical. Recombination of the two radicals to a cyclopropane
full valence compound is only possible when the triplet state converts to the higher energy singlet state first.
The most common use of the TMM framework is in transition metal chemistry
and specifically the use in trimethylenemethane cycloaddition
reaction. In 1979, Trost et al. published a palladium catalyzed [3+2] cycloaddition
of trimethylenemethane.
Other studied biradicals are those based on Pleiadene , extended viologens
, corannulene
s , nitronyl-nitroxide , bis(phenalenyl)s and teranthenes .
Pleiadene has been synthesised from acenaphthylene
and anthranilic acid
/ amyl nitrite
:
is postulated to occur in ring opening of cyclopropanones, allene oxides and in the Favorskii rearrangement
. The intermediate has been produced by reaction of oxygen radical anions with acetone
and studied by photoelectron spectroscopy . The experimental electron affinity
of OXA is 1.94 eV.
s (NBMOs).
Both NBMOs of molecules with non-disjoint characteristics such as trimethylenemethane (TMM) have electron density
at the same atom
. According to Hund's rule, each orbital is filled with one electron with parallel spin, avoiding the Coulomb repulsion
by filling one orbital with two electrons. Therefore, such molecules with non-disjoint NBMOs are expected to prefer a triplet
ground state
.
In contrast, the NBMOs of the molecules with disjoint characteristics such as tetramethyleneethane (TME) can be described without having electron density at the same atom. With such MOs, the destabilization factor by the Coulomb repulsion becomes much smaller than with non-disjoint type molecules, and therefore the relative stability of the singlet
ground state to the triplet ground state will be nearly equal, or even reversed because of exchange interaction
.
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
that cannot be assigned classical Kekulé structures. Since non-Kekulé molecules have two or more formal radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
centers, their spin-spin
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
interactions can cause electrical conductivity or ferromagnetism
Ferromagnetism
Ferromagnetism is the basic mechanism by which certain materials form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished...
(molecule-based magnets), and applications to functional materials are expected. However, as these molecules are quite reactive and most of them are easily decomposed or polymerized
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
at room temperature, strategies for stabilization are needed for their practical use. Synthesis and observation of these reactive molecules are generally accomplished by matrix-isolation methods.
The simplest non-Kekulé molecules are biradicals.
Biradicals
A biradical in organic chemistryOrganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is an even-electron chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with two free radical centres which act independently of each other and not to be confused with diradical
Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
s in general.
One of the first biradicals was synthesized by Wilhelm Schlenk
Wilhelm Schlenk
Wilhelm Johann Schlenk was a German chemist. He was born in Munich and also studied chemistry there. Schlenk succeeded Hermann Emil Fischer at the University of Berlin in 1919....
in 1915 following the same methodology as Moses Gomberg
Moses Gomberg
Moses Gomberg was a chemistry professor at the University of Michigan....
's triphenylmethyl radical
Triphenylmethyl radical
The triphenylmethyl radical is a persistent radical and the first-ever radical described in organic chemistry. It can be prepared by homolysis of triphenylmethyl chloride 1 by a metal like silver or zinc in benzene or diethyl ether. The radical 2 forms a chemical equilibrium with the quinoid type...
. The so-called Schlenk-Brauns hydrocarbons are:

Triplet state
A spin triplet is a set of three quantum states of a system, each with total spin S = 1 . The system could consist of a single elementary massive spin 1 particle such as a W or Z boson, or be some multiparticle state with total spin angular momentum of one.In physics, spin is the angular momentum...
.
Other classic biradicals are those synthesed by Tschitschibabin , Yang and Coppinger .
| Tschitschibabin biradical (1907) | Yang biradical (1960) | Coppinger biradical 1962 | ||
Trimethylenemethane
A well studied biradical is trimethylenemethane or TMM:
Diazo
Diazo refers to a type of organic compound called diazo compound that has two linked nitrogen atoms as a terminal functional group. The general formula is R2C=N2. The simplest example of a diazo compound is diazomethane...
precursor with expulsion of nitrogen or from photolysis of 2-methylenecyclobutanone with expulsion of carbon monoxide. In 1966 Paul Dowd determined with electron spin resonance that this compound also has a triplet state
Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
. In a crystalline host the 6 hydrogen atoms in TMM are identical. Recombination of the two radicals to a cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
full valence compound is only possible when the triplet state converts to the higher energy singlet state first.
The most common use of the TMM framework is in transition metal chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
and specifically the use in trimethylenemethane cycloaddition
Trimethylenemethane cycloaddition
Trimethylenemethane cycloaddition is the formal [3+2] annulation of trimethylenemethane derivatives to two-atom pi systems. Although TMM itself is too reactive and unstable to be stored, reagents that can generate TMM or TMM synthons in situ can be used to effect cycloaddition reactions with...
reaction. In 1979, Trost et al. published a palladium catalyzed [3+2] cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
of trimethylenemethane.
Quinodimethanes & PAH's
Non-Kekulé quinodimethanes are biradicaloids of a six-membered ring with methylene substituents. Non-Kekulé polynuclear aromatics are composed of several fused six-membered rings. The synthesis of Triangulene, the simplest non-Kekulé polynuclear aromatic, was first tried by Eric Clar in 1953, but it was not even observed until the syntheses of trioxytriangulene by Richard J. Bushby in 1995 and kinetically stabilized triangulene by Kazuhiro Nakasuji in 2001. A related class of biradicals are para-benzynes.Other studied biradicals are those based on Pleiadene , extended viologens
Viologen
Viologens are toxic bipyridinium derivatives of 4,4'-bipyridyl. The name is because this class of compounds is easily reduced to the radical mono cation, which is intensely blue coloured....
, corannulene
Corannulene
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is [5]circulene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of...
s , nitronyl-nitroxide , bis(phenalenyl)s and teranthenes .
| Teranthene biradical Singlet. max. 3 stabilizing Clar sextets, stable rt, air. 50% biradical, molecular section of graphene Graphene Graphene is an allotrope of carbon, whose structure is one-atom-thick planar sheets of sp2-bonded carbon atoms that are densely packed in a honeycomb crystal lattice. The term graphene was coined as a combination of graphite and the suffix -ene by Hanns-Peter Boehm, who described single-layer... |
Bisphenalenyl biradical Singlet. max. 6 stabilizing Clar sextets, stable rt, air. 42% biradical | |
Pleiadene has been synthesised from acenaphthylene
Acenaphthylene
Acenaphthylene is a polycyclic aromatic hydrocarbon consisting of naphthalene with an ethylene bridge connecting positions 1 and 8. It is a constituent of coal tar. Reduction of the ethylene group gives the related compound acenaphthene. Unlike most polycyclic aromatic hydrocarbons, it has no...
and anthranilic acid
Anthranilic acid
Anthranilic acid is the organic compound with the formula C6H4COOH. This amino acid is a white solid when pure, although commercial samples may appear yellow. The molecule consists of a benzene ring with two adjacent functional groups, a carboxylic acid and an amine...
/ amyl nitrite
Amyl nitrite
Amyl nitrite is the chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrito functional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group...
:
| Pleiadene generation and dimerization |
Oxyallyl
In the oxyallyl diradical (OXA) one methylene group in TMM is replaced by oxygen. This reactive intermediateReactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
is postulated to occur in ring opening of cyclopropanones, allene oxides and in the Favorskii rearrangement
Favorskii rearrangement
The Favorskii rearrangement , named for the Russian chemist Alexei Yevgrafovich Favorskii, is most principally a rearrangement of cyclopropanones and α-halo ketones which leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorski rearrangement constitutes a ring...
. The intermediate has been produced by reaction of oxygen radical anions with acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
and studied by photoelectron spectroscopy . The experimental electron affinity
Electron affinity
The Electron affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion....
of OXA is 1.94 eV.
Classification
Non-Kekulé molecules with two formal radical centers (non-Kekulé diradicals) can be classified into non-disjoint and disjoint by the shape of their two non-bonding molecular orbitalMolecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
s (NBMOs).
Both NBMOs of molecules with non-disjoint characteristics such as trimethylenemethane (TMM) have electron density
Electron density
Electron density is the measure of the probability of an electron being present at a specific location.In molecules, regions of electron density are usually found around the atom, and its bonds...
at the same atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
. According to Hund's rule, each orbital is filled with one electron with parallel spin, avoiding the Coulomb repulsion
Coulomb's law
Coulomb's law or Coulomb's inverse-square law, is a law of physics describing the electrostatic interaction between electrically charged particles. It was first published in 1785 by French physicist Charles Augustin de Coulomb and was essential to the development of the theory of electromagnetism...
by filling one orbital with two electrons. Therefore, such molecules with non-disjoint NBMOs are expected to prefer a triplet
Triplet state
A spin triplet is a set of three quantum states of a system, each with total spin S = 1 . The system could consist of a single elementary massive spin 1 particle such as a W or Z boson, or be some multiparticle state with total spin angular momentum of one.In physics, spin is the angular momentum...
ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
.
In contrast, the NBMOs of the molecules with disjoint characteristics such as tetramethyleneethane (TME) can be described without having electron density at the same atom. With such MOs, the destabilization factor by the Coulomb repulsion becomes much smaller than with non-disjoint type molecules, and therefore the relative stability of the singlet
Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
ground state to the triplet ground state will be nearly equal, or even reversed because of exchange interaction
Exchange interaction
In physics, the exchange interaction is a quantum mechanical effect without classical analog which increases or decreases the expectation value of the energy or distance between two or more identical particles when their wave functions overlap...
.

