
Mupirocin
Encyclopedia
Mupirocin is an antibiotic
originally isolated from Pseudomonas fluorescens
NCIMB 10586, developed by Beecham
.
Mupirocin is bacteriostatic at low concentrations and bactericidal at high concentrations. It is used topically and is effective against Gram-positive
bacteria, including MRSA
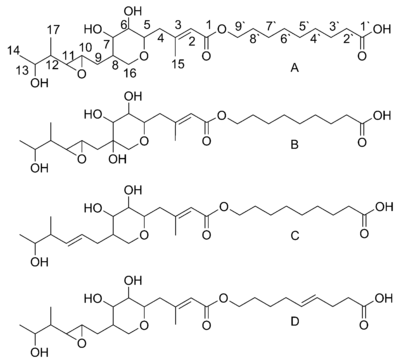
. Mupirocin is a mixture of several pseudomonic acids, with pseudomonic acid A (PA-A) constituting greater than 90% of the mixture. Also present in mupirocin are pseudomonic acid B with an additional hydroxyl group at C8, pseudomonic acid C with a double bond between C10 and C11, instead of the epoxide of PA-A, and pseudomonic acid D with a double bond at C4` and C5` in the 9-hydroxy-nonanoic acid portion of mupirocin.
and RNA
synthesis in Staphylococcus aureus
while DNA
and cell wall formation were also negatively impacted to a lesser degree. The inhibition of RNA synthesis was shown to be a protective mechanism in response to a lack of one amino acid
, isoleucine
. In vivo studies in Escherichia coli
demonstrated that pseudomonic acid inhibits isoleucine t-RNA synthetase (IleRS). This mechanism of action is shared with furanomycin
, an analog of isoleucine.
, open wounds, etc. It is also useful in the treatment of methicillin-resistant Staphylococcus aureus
(MRSA), which is a significant cause of death in hospitalized patients having received systemic antibiotic therapy. It is suggested, however, that mupirocin cannot be used for extended periods of time, or indiscriminately, as resistance does develop, and could, if it becomes widespread, destroy mupirocin's value as a treatment for MRSA. It may also result in overgrowth of non-susceptible organisms.
to mupirocin emerged. Two distinct populations of mupirocin-resistant S. aureus were isolated. One strain possessed low-level resistance, MuL, (MIC = 8-256 mg/L) and another possessed high-level resistance, MuH, (MIC > 256 mg/L). Resistance in the MuL strains is probably due to mutation
s in the organism's wild-type isoleucinyl-tRNA synthetase. In E. coli IleRS, a single amino acid mutation was shown to alter mupirocin resistance. MuH is linked to the acquisition of a separate Ile synthetase gene, mupA. Mupirocin is not a viable antibiotic against MuH strains. Other antibiotic agents such as azelaic acid
, nitrofurazone
, silver sulfadiazine, and ramoplanin
have been shown to be effective against MuH strains.
The mechanism of mupirocin differs from other clinical antibiotics, rendering cross-resistance to other antibiotics unlikely. However, the MupA gene may co-transfer with other antibacterial resistance genes. This has been observed already with resistance genes for triclosan
, tetracycline, and trimethoprim
.

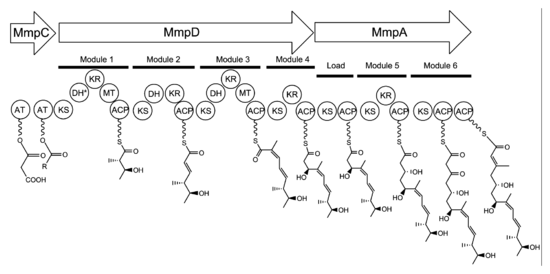
contains six multi-domain
enzymes and twenty-six other peptides (Table 1). Four large multi-domain type I polyketide synthase
(PKS) proteins are encoded, as well as several single function enzymes with sequence similarity to type II PKSs. Therefore, it is believed that mupirocin is constructed by a mixed type I and type II PKS system. The mupirocin cluster exhibits an atypical acyltransferase
(AT) organization, in that there are only two AT domains, and both are found on the same protein, MmpC. These AT domains are the only domains present on MmpC, while the other three type I PKS proteins contain no AT domains. The mupirocin pathway also contains several tandem acyl carrier protein
doublets or triplets. This may be an adaptation to increase the throughput rate or to bind multiple substrates simultaneously.
Pseudomonic acid A is the product of an esterification between the 17C polyketide monic acid and the 9C fatty acid
9-hydroxy-nonanoic acid. The possibility that the entire molecule is assembled as a single polyketide with a Baeyer-Villiger oxidation inserting an oxygen
into the carbon backbone has been ruled out because C1 of monic acid and C9' of 9-hydroxy-nonanoic acid are both derived from C1 of acetate.
Assembly of monic acid is continued by the transfer of the 12C product of MmpD to MmpA. Two more rounds of extension with malonyl-CoA units are achieved by module 5 and 6. Module 5 also contains a KR domain.
The formation of the pyran
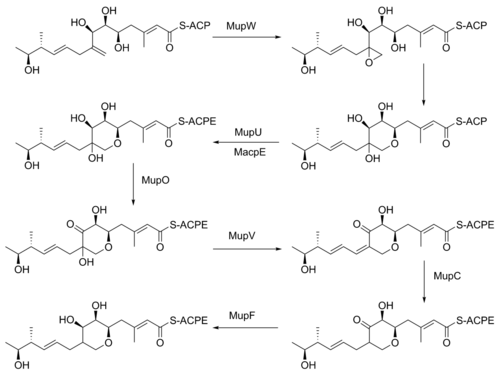
ring requires many enzyme-mediated steps (Figure 4). The double bond between C8 and C9 is proposed to migrate to between C8 and C16. Gene knockout
experiments of mupO, mupU, mupV, and macpE have eliminated PA-A production. PA-B production is not removed by these knockouts, demonstrating that PA-B is not created by hydroxylating PA-A. A knockout of mupW eliminated the pyran ring, identifying MupW as being involved in ring formation. It is not known whether this occurs before or after the esterification of monic acid to 9-hydroxy-nonanoic acid.
The epoxide
of PA-A at C10-11 is believed to be inserted after pyran formation by a cytochrome P450 such as MupO. A gene knockout of mupO abolished PA-A production but PA-B, which also conatins the C10-C11 epoxide, remained. This indicates that MupO is either not involved or is not essential for this epoxidation step.
feeding has shown that C1-C6 are constructed with acetate in the canonical fashion of fatty acid synthesis
. C7' shows only C1 labeling of acetate, while C8' and C9' show a reversed pattern of 13C labeled acetate. It is speculated that C7-C9 arises from a 3-hydroxypropionate starter unit, which is extended three times with malonyl-CoA and fully reduced to yield 9-HN. It has also been suggested that 9-HN is initiated by 3-hydroxy-3-methylglutaric acid (HMG). This latter theory was not supported by feeding of [3-14C] or [3,6-13C2]-HMG.
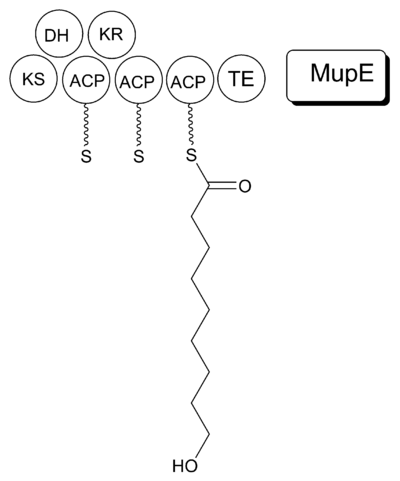
It is proposed that MmpB to catalyzes the synthesis of 9-HN (Figure 5). MmpB contains a KS, KR, DH, 3 ACPs, and a thioesterase (TE) domain. It does not contain an enoyl reductase (ER) domain, which would be required for the complete reduction to the nine-carbon fatty acid. MupE is a single-domain protein that shows sequence similarity to known ER domains and may complete the reaction. It also remains possible that 9-hydroxy-nonanoic acid is derived partially or entirely from outside of the mupirocin cluster.
Antibiotic
An antibacterial is a compound or substance that kills or slows down the growth of bacteria.The term is often used synonymously with the term antibiotic; today, however, with increased knowledge of the causative agents of various infectious diseases, antibiotic has come to denote a broader range of...
originally isolated from Pseudomonas fluorescens
Pseudomonas fluorescens
Pseudomonas fluorescens is a common Gram-negative, rod-shaped bacterium. It belongs to the Pseudomonas genus; 16S rRNA analysis has placed P. fluorescens in the P. fluorescens group within the genus, to which it lends its name....
NCIMB 10586, developed by Beecham
Beecham (pharmaceutical company)
Beecham was a British pharmaceutical company. It was once a constituent of the FTSE 100 Index. Beecham, after having merged with SmithKline Beckman, merged with GlaxoWellcome to become GlaxoSmithKline .-History:...
.
Mupirocin is bacteriostatic at low concentrations and bactericidal at high concentrations. It is used topically and is effective against Gram-positive
Gram-positive
Gram-positive bacteria are those that are stained dark blue or violet by Gram staining. This is in contrast to Gram-negative bacteria, which cannot retain the crystal violet stain, instead taking up the counterstain and appearing red or pink...
bacteria, including MRSA
Methicillin-resistant Staphylococcus aureus
Methicillin-resistant Staphylococcus aureus is a bacterium responsible for several difficult-to-treat infections in humans. It is also called multidrug-resistant Staphylococcus aureus and oxacillin-resistant Staphylococcus aureus...
. Mupirocin is a mixture of several pseudomonic acids, with pseudomonic acid A (PA-A) constituting greater than 90% of the mixture. Also present in mupirocin are pseudomonic acid B with an additional hydroxyl group at C8, pseudomonic acid C with a double bond between C10 and C11, instead of the epoxide of PA-A, and pseudomonic acid D with a double bond at C4` and C5` in the 9-hydroxy-nonanoic acid portion of mupirocin.
Mechanism
Mupirocin has been shown to strongly inhibit proteinProtein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
and RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
synthesis in Staphylococcus aureus
Staphylococcus aureus
Staphylococcus aureus is a facultative anaerobic Gram-positive coccal bacterium. It is frequently found as part of the normal skin flora on the skin and nasal passages. It is estimated that 20% of the human population are long-term carriers of S. aureus. S. aureus is the most common species of...
while DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
and cell wall formation were also negatively impacted to a lesser degree. The inhibition of RNA synthesis was shown to be a protective mechanism in response to a lack of one amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
, isoleucine
Isoleucine
Isoleucine is an α-amino acid with the chemical formula HO2CCHCHCH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA....
. In vivo studies in Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
demonstrated that pseudomonic acid inhibits isoleucine t-RNA synthetase (IleRS). This mechanism of action is shared with furanomycin
Furanomycin
Furanomycin is an isoleucine analog....
, an analog of isoleucine.
Uses
Mupirocin is used as a topical treatment for bacterial skin infections, for example, furuncle, impetigoImpetigo
Impetigo is a highly contagious bacterial skin infection most common among pre-school children. People who play close contact sports such as rugby, American football and wrestling are also susceptible, regardless of age. Impetigo is not as common in adults. The name derives from the Latin impetere...
, open wounds, etc. It is also useful in the treatment of methicillin-resistant Staphylococcus aureus
Methicillin-resistant Staphylococcus aureus
Methicillin-resistant Staphylococcus aureus is a bacterium responsible for several difficult-to-treat infections in humans. It is also called multidrug-resistant Staphylococcus aureus and oxacillin-resistant Staphylococcus aureus...
(MRSA), which is a significant cause of death in hospitalized patients having received systemic antibiotic therapy. It is suggested, however, that mupirocin cannot be used for extended periods of time, or indiscriminately, as resistance does develop, and could, if it becomes widespread, destroy mupirocin's value as a treatment for MRSA. It may also result in overgrowth of non-susceptible organisms.
Resistance
Shortly after the clinical use of Mupirocin began, strains of Staphylococcus aureus that were resistantDrug resistance
Drug resistance is the reduction in effectiveness of a drug such as an antimicrobial or an antineoplastic in curing a disease or condition. When the drug is not intended to kill or inhibit a pathogen, then the term is equivalent to dosage failure or drug tolerance. More commonly, the term is used...
to mupirocin emerged. Two distinct populations of mupirocin-resistant S. aureus were isolated. One strain possessed low-level resistance, MuL, (MIC = 8-256 mg/L) and another possessed high-level resistance, MuH, (MIC > 256 mg/L). Resistance in the MuL strains is probably due to mutation
Mutation
In molecular biology and genetics, mutations are changes in a genomic sequence: the DNA sequence of a cell's genome or the DNA or RNA sequence of a virus. They can be defined as sudden and spontaneous changes in the cell. Mutations are caused by radiation, viruses, transposons and mutagenic...
s in the organism's wild-type isoleucinyl-tRNA synthetase. In E. coli IleRS, a single amino acid mutation was shown to alter mupirocin resistance. MuH is linked to the acquisition of a separate Ile synthetase gene, mupA. Mupirocin is not a viable antibiotic against MuH strains. Other antibiotic agents such as azelaic acid
Azelaic acid
Azelaic acid is an organic compound with the formula 72. This saturated dicarboxylic acid exists as a white powder. It is found in wheat, rye, and barley. It is a component of a number of hair and skin conditioners.-Production:...
, nitrofurazone
Nitrofurazone
Nitrofurazone, 2-hydrazinecarboxamide, chemical formula C6H6N4O4, is a pale yellow crystalline compound.This bactericidal compound is used as an antibiotic most commonly in the form of ointments...
, silver sulfadiazine, and ramoplanin
Ramoplanin
Ramoplanin is a glycolipodepsipeptide antibiotic drug derived from strain ATCC 33076 of Actinoplanes.-Mechanism:It exerts its bacteriocidal effect by inhibiting cell wall biosynthesis, acting by inhibiting the transglycosylation step of peptidoglycan synthesis.-Uses:Its development has been...
have been shown to be effective against MuH strains.
The mechanism of mupirocin differs from other clinical antibiotics, rendering cross-resistance to other antibiotics unlikely. However, the MupA gene may co-transfer with other antibacterial resistance genes. This has been observed already with resistance genes for triclosan
Triclosan
Triclosan is an antibacterial and antifungal agent. It is a polychloro phenoxy phenol. Despite being used in many consumer products, beyond its use in toothpaste to prevent gingivitis, there is no evidence according to the American Food and Drug Administration that triclosan provides an extra...
, tetracycline, and trimethoprim
Trimethoprim
Trimethoprim is a bacteriostatic antibiotic mainly used in the prophylaxis and treatment of urinary tract infections.It belongs to the class of chemotherapeutic agents known as dihydrofolate reductase inhibitors...
.
Biosynthesis






Biosynthesis of Pseudomonic Acid A
The 74 kb mupirocin gene clusterGene cluster
A gene cluster is a set of two or more genes that serve to encode for the same or similar products. Because populations from a common ancestor tend to possess the same varieties of gene clusters, they are useful for tracing back recent evolutionary history...
contains six multi-domain
Protein domain
A protein domain is a part of protein sequence and structure that can evolve, function, and exist independently of the rest of the protein chain. Each domain forms a compact three-dimensional structure and often can be independently stable and folded. Many proteins consist of several structural...
enzymes and twenty-six other peptides (Table 1). Four large multi-domain type I polyketide synthase
Polyketide synthase
Polyketide synthases are a family of multi-domain enzymes or enzyme complexes that produce polyketides, a large class of secondary metabolites, in bacteria, fungi, plants, and a few animal lineages...
(PKS) proteins are encoded, as well as several single function enzymes with sequence similarity to type II PKSs. Therefore, it is believed that mupirocin is constructed by a mixed type I and type II PKS system. The mupirocin cluster exhibits an atypical acyltransferase
Acyltransferase
Acyltransferase is a type of transferase enzyme that acts upon acyl groups.Examples include:* Glyceronephosphate O-acyltransferase* Lecithin-cholesterol acyltransferase...
(AT) organization, in that there are only two AT domains, and both are found on the same protein, MmpC. These AT domains are the only domains present on MmpC, while the other three type I PKS proteins contain no AT domains. The mupirocin pathway also contains several tandem acyl carrier protein
Acyl carrier protein
The acyl carrier protein is an important component in both fatty acid and polyketide biosynthesis with the growing chain bound during synthesis as a thiol ester at the distal thiol of a 4'-phosphopantethiene moiety...
doublets or triplets. This may be an adaptation to increase the throughput rate or to bind multiple substrates simultaneously.
Pseudomonic acid A is the product of an esterification between the 17C polyketide monic acid and the 9C fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
9-hydroxy-nonanoic acid. The possibility that the entire molecule is assembled as a single polyketide with a Baeyer-Villiger oxidation inserting an oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
into the carbon backbone has been ruled out because C1 of monic acid and C9' of 9-hydroxy-nonanoic acid are both derived from C1 of acetate.
Monic acid biosynthesis
Biosynthesis of the 17C monic acid unit begins on MmpD (Figure 1). One of the AT domains from MmpC may transfer an activated acetyl group from acetyl-Coenzyme A (CoA) to the first ACP domain. The chain is extended by malonyl-CoA, followed by a SAM-dependent methylation at C12 (see Figure 2 for PA-A numbering) and reduction of the B-keto group to an alcohol. The dehydration (DH) domain in module 1 is predicted to be non-functional due to a mutation in the conserved active site region. Module 2 adds another two carbons by malonyl-CoA extender unit, followed by ketoreduction (KR) and dehydration. Module three adds a malonyl-CoA extender unit, followed by SAM-dependent methylation at C8, ketoreduction, and dehydration. Module 4 extends the molecule with a malonyl-CoA unit followed by ketoreduction.Assembly of monic acid is continued by the transfer of the 12C product of MmpD to MmpA. Two more rounds of extension with malonyl-CoA units are achieved by module 5 and 6. Module 5 also contains a KR domain.
Post-PKS tailoring
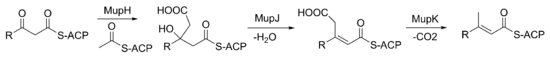
The keto group at C3 is replaced with a methyl group in a multi-step reaction (Figure 3). MupG begins by decarboxylating a malonyl-ACP. The alpha carbon of the resulting acetyl-ACP is linked to C3 of the polyketide chain by MupH. This intermediate is dehydrated and decarboxylated by MupJ and MupK, respectively.The formation of the pyran
Pyran
In chemistry, a pyran, or oxine, is a six-membered heterocyclic, non-aromatic ring, consisting of five carbon atoms and one oxygen atom and containing two double bonds. The molecular formula is C5H6O. There are two isomers of pyran that differ by the location of the double bonds...
ring requires many enzyme-mediated steps (Figure 4). The double bond between C8 and C9 is proposed to migrate to between C8 and C16. Gene knockout
Gene knockout
A gene knockout is a genetic technique in which one of an organism's genes is made inoperative . Also known as knockout organisms or simply knockouts, they are used in learning about a gene that has been sequenced, but which has an unknown or incompletely known function...
experiments of mupO, mupU, mupV, and macpE have eliminated PA-A production. PA-B production is not removed by these knockouts, demonstrating that PA-B is not created by hydroxylating PA-A. A knockout of mupW eliminated the pyran ring, identifying MupW as being involved in ring formation. It is not known whether this occurs before or after the esterification of monic acid to 9-hydroxy-nonanoic acid.
The epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
of PA-A at C10-11 is believed to be inserted after pyran formation by a cytochrome P450 such as MupO. A gene knockout of mupO abolished PA-A production but PA-B, which also conatins the C10-C11 epoxide, remained. This indicates that MupO is either not involved or is not essential for this epoxidation step.
9-Hydroxy-nonanoic acid biosynthesis
The nine-carbon fatty acid 9-hydroxy-nonanoic acid (9-HN) is derived as a separate compound and later esterified to monic acid to form pseudomonic acid. 13C labeled acetateAcetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
feeding has shown that C1-C6 are constructed with acetate in the canonical fashion of fatty acid synthesis
Fatty acid synthesis
Fatty acid synthesis is the creation of fatty acids from acetyl-CoA and malonyl-CoA precursors through action of enzymes called fatty acid synthases...
. C7' shows only C1 labeling of acetate, while C8' and C9' show a reversed pattern of 13C labeled acetate. It is speculated that C7-C9 arises from a 3-hydroxypropionate starter unit, which is extended three times with malonyl-CoA and fully reduced to yield 9-HN. It has also been suggested that 9-HN is initiated by 3-hydroxy-3-methylglutaric acid (HMG). This latter theory was not supported by feeding of [3-14C] or [3,6-13C2]-HMG.
It is proposed that MmpB to catalyzes the synthesis of 9-HN (Figure 5). MmpB contains a KS, KR, DH, 3 ACPs, and a thioesterase (TE) domain. It does not contain an enoyl reductase (ER) domain, which would be required for the complete reduction to the nine-carbon fatty acid. MupE is a single-domain protein that shows sequence similarity to known ER domains and may complete the reaction. It also remains possible that 9-hydroxy-nonanoic acid is derived partially or entirely from outside of the mupirocin cluster.
External links
- Bactroban Prescribing Information GlaxoSmithKline
- Bactroban Nasal Prescribing Information GlaxoSmithKline

