
Lehmstedt-Tanasescu reaction
Encyclopedia
The Lehmstedt-Tanasescu reaction is a method in organic chemistry
for the organic synthesis
of acridone
derivatives (3) from a 2-nitrobenzaldehyde
(1) and an arene compound (2):
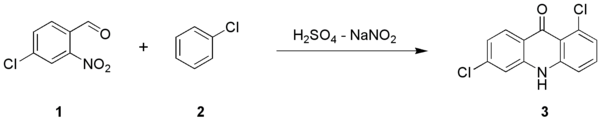 The reaction is named after two chemist
The reaction is named after two chemist
s who devoted part of their careers to research into this synthetic method, the German chemist Kurt Lehmstedt and the Romanian chemist Ioan Tănăsescu
. Variations to the reaction name are the Lehmsted-Tănăsescu-reaction, Lehmsted-Tănăsescu-acridone-synthesis and Lehmsted-Tanasescu-acridone-synthesis.
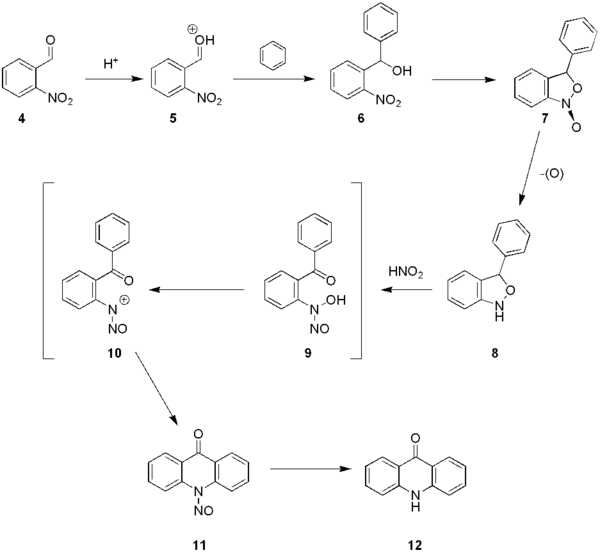
the precursor molecule 2-nitrobenzaldehyde 4 is protonated, often by sulfuric acid
, to intermediate 5, followed by an electrophilic
attack to benzene
(other arenes can be used as well). The resulting benzhydrol 6 cyclisizes to 7 and finally to compound 8. Treatment of this intermediate with nitrous acid
(sodium nitrite
en sulfuric acid) leads to the N-nitroso
acridone 11 via intermediates 9 en 10. The N-nitroso group is removed by an acid
in the final step. The procedure is an example of a one-pot synthesis
.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
for the organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
of acridone
Acridone
Acridone is an organic compound based on the acridine skeleton, with a carbonyl group at the 9 position. It may be synthesized by the self-condensation of N-phenylanthranilic acid.-Derivatives:...
derivatives (3) from a 2-nitrobenzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
(1) and an arene compound (2):

Chemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
s who devoted part of their careers to research into this synthetic method, the German chemist Kurt Lehmstedt and the Romanian chemist Ioan Tănăsescu
Ioan Tanasescu
Ioan Tănăsescu was a Romanian chemist.He discovered the Lehmstedt-Tanasescu reaction, which was improved by Karl Lehmstedt. He studied at the University of Bucharest and the University of Cluj....
. Variations to the reaction name are the Lehmsted-Tănăsescu-reaction, Lehmsted-Tănăsescu-acridone-synthesis and Lehmsted-Tanasescu-acridone-synthesis.
Reaction mechanism
In the first step of the reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
the precursor molecule 2-nitrobenzaldehyde 4 is protonated, often by sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
, to intermediate 5, followed by an electrophilic
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
attack to benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
(other arenes can be used as well). The resulting benzhydrol 6 cyclisizes to 7 and finally to compound 8. Treatment of this intermediate with nitrous acid
Nitrous acid
Nitrous acid is a weak and monobasic acid known only in solution and in the form of nitrite salts.Nitrous acid is used to make diazides from amines; this occurs by nucleophilic attack of the amine onto the nitrite, reprotonation by the surrounding solvent, and double-elimination of water...
(sodium nitrite
Sodium nitrite
Sodium nitrite is the inorganic compound with the chemical formula NaNO2. It is a white to slight yellowish crystalline powder that is very soluble in water and is hygroscopic...
en sulfuric acid) leads to the N-nitroso
Nitroso
Nitroso refers to a functional group in organic chemistry which has the general formula RNO. Nitroso compounds are a class of organic compounds containing the nitroso functional group, R−N=O....
acridone 11 via intermediates 9 en 10. The N-nitroso group is removed by an acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
in the final step. The procedure is an example of a one-pot synthesis
One-pot synthesis
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction whereby a reactant is subjected to successive chemical reactions in just one reactor...
.


