Knorr pyrrole synthesis
Encyclopedia
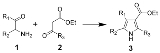
The Knorr pyrrole synthesis is a widely used chemical reaction
that synthesizes substituted pyrrole
s (3). The method involves the reaction of an α-amino-ketone
(1) and a compound containing a methylene group α- to (bonded to the next carbon to) a carbonyl group (2).

Because α-amino-ketones self-condense very easily, they must be prepared in situ. The usual way of doing this is from the relevant oxime
.
The original Knorr synthesis employed two equivalents of ethyl acetoacetate
, one of which was converted to ethyl 2-oximinoacetoacetate by dissolving it in glacial acetic acid, and slowly adding one equivalent of saturated aqueous sodium nitrite
, under external cooling. Zinc
dust was then stirred in, reducing the oxime
group to the amine. This reduction consumes two equivalents of zinc and four equivalents of acetic acid.
Modern practice is to add the oxime solution resulting from the nitrosation and the zinc dust gradually to a well-stirred solution of ethyl acetoacetate in glacial acetic acid. The reaction is exothermic
, and the mixture can reach the boiling point, if external cooling is not applied. The resulting product, diethyl 3,5-dimethylpyrrole-2,4-dicarboxylate, has been called Knorr's Pyrrole ever since. In the Scheme above, R2 = COOEt, and R1 = R3 = Me represent this original reaction.
Knorr's pyrrole can be derivatized in a number of useful manners. One equivalent of sodium hydroxide will saponify the 2-ester selectively. Dissolving Knorr's Pyrrole in concentrated sulfuric acid
, and then pouring the resulting solution into water will hydrolyze the 4-ester group selectively. The 5-methyl group can be variously oxidized to chloromethyl, aldehyde, or carboxylic acid functionality by the use of stoichiometric sulfuryl chloride
in glacial acetic acid. Alternatively, the nitrogen atom can be alkylated. The two ester positions can be more smoothly differentiated by incorporating benzyl
or tertiary-butyl groups via the corresponding acetoacetate esters. Benzyl groups can be removed by catalytic hydrogenolysis
over palladium on carbon
, and tertiary-butyl groups can be removed by treatment with trifluoroacetic acid
, or boiling aqueous acetic acid. R1 and R3 (as well as R2 and "Et") can be varied by the application of appropriate beta-ketoesters readily made by a synthesis emanating from acid chlorides, Meldrum's acid
, and the alcohol of one's choice. Ethyl and benzyl esters are easily made thereby, and the reaction is noteworthy in that even the highly hindered tertiary-butyl alcohol gives very high yields in this synthesis.
Levi and Zanetti extended the Knorr synthesis in 1894 to the use of acetylacetone
(2,4-pentanedione) in reaction with ethyl 2-oximinoacetoacetate. The result was ethyl 4-acetyl-3,5-dimethylpyrrole-2-carboxylate, where "OEt" = R1 = R3 = Me, and R2 = COOEt. The 4-acetyl group could easily be reduced to a 4-ethyl group by use of the Wolff-Kishner reduction
(hydrazine and alkali, heated); hydrogenolysis, or the use of diborane
. Benzyl or tertiary-butyl acetoacetates also work well in this system, and with close temperature control, the tertiary-butyl system gives a very high yield (close to 80%). N,N-dialkyl pyrrole-2- and/or 4-carboxamides may be prepared by the use of N,N-dialkyl acetoacetamides in the synthesis. Even thioesters have been successfully prepared, using the method. As for the nitrosation of beta-ketoesters, despite the numerous literature specifications of tight temperature control on the nitrosation, the reaction behaves almost like a titration, and the mixture can be allowed to reach even 40 degrees Celsius without significantly impacting the final yield.
Fischer and Fink found that Zanetti's synthesis from 2,4-pentanedione and ethyl 2-oximinoacetoacetate gave ethyl 3,5-dimethylpyrrole-2-carboxylate as a trace byproduct. Similarly, 3-ketobutyraldehyde diethyl acetal led to the formation of ethyl 5-methylpyrrole-2-carboxylate. Both of these products resulted from the loss of the acetyl group from the inferred ethyl 2-aminoacetoacetate intermediate. An important product of the Fischer-Fink synthesis was ethyl 4,5-dimethylpyrrole-2-carboxylate, made from ethyl 2-oximinoacetoacetate and 2-methyl-3-oxobutanal, in turn made by the Claisen condensation
of 2-butanone with ethyl formate
.,
G.G. Kleinspehn reported that the Fischer-Fink connectivity could be forced to occur exclusively, by the use of diethyl oximinomalonate in the synthesis, with 2,4-pentanedione, or its 3-alkyl substituted derivatives. Yields were high, around 60%, and this synthesis eventually came to be one of the most important in the repertory. Yields were significantly improved, by the use of preformed diethyl aminomalonate (prepared by the hydrogenolysis of diethyl oximinomalonate in ethanol, over Pd/C), and adding a mixture of diethyl aminomalonate and the beta-diketone to actively boiling glacial acetic acid.
Meanwhile, Johnson had extended the Fischer-Fink synthesis by reacting 2-oximinoacetoacetate esters (ethyl, benzyl, or tertiary-butyl), with 3-alkyl substituted 2,4-pentanediones. Others extended the Kleinspehn synthesis by the use of unsymmetrical beta-diketones (such as 3-alkyl substituted 2,4-hexanediones), which preferentially reacted initially at the less hindered acetyl group and afforded the corresponding 5-methylpyrrole-2-carboxylate esters. N,N-Dialkyl 2-oximinoacetoacetamides also were found to give pyrroles when reacted under Knorr conditions with 3-substituted-2,4-pentanediones, in yields comparable to the corresponding esters (around 45%). However, when unsymmetrical diketones were used, it was found that the acetyl group from the acetoacetamide was retained in the product, and one of the acyl groups from the diketone had been lost. This same mechanism occurs to a minor extent in the acetoacetate ester systems, and had previously been detected radiochemically by Harbuck and Rapoport. Most of the above-described syntheses have application in the synthesis of porphyrins, bile pigments, and dipyrrins.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
that synthesizes substituted pyrrole
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
s (3). The method involves the reaction of an α-amino-ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
(1) and a compound containing a methylene group α- to (bonded to the next carbon to) a carbonyl group (2).

Method
The mechanism requires zinc and acetic acid as catalysts. It will proceed at room temperature.Because α-amino-ketones self-condense very easily, they must be prepared in situ. The usual way of doing this is from the relevant oxime
Oxime
An oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
.
The original Knorr synthesis employed two equivalents of ethyl acetoacetate
Ethyl acetoacetate
The organic compound ethyl acetoacetate is the ethyl ester of acetoacetic acid. It is mainly used as a chemical intermediate in the production of a wide variety of compounds, such as amino acids, analgesics, antibiotics, antimalarial agents, antipyrine and aminopyrine, and vitamin B1; as well as...
, one of which was converted to ethyl 2-oximinoacetoacetate by dissolving it in glacial acetic acid, and slowly adding one equivalent of saturated aqueous sodium nitrite
Sodium nitrite
Sodium nitrite is the inorganic compound with the chemical formula NaNO2. It is a white to slight yellowish crystalline powder that is very soluble in water and is hygroscopic...
, under external cooling. Zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
dust was then stirred in, reducing the oxime
Oxime
An oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
group to the amine. This reduction consumes two equivalents of zinc and four equivalents of acetic acid.
Modern practice is to add the oxime solution resulting from the nitrosation and the zinc dust gradually to a well-stirred solution of ethyl acetoacetate in glacial acetic acid. The reaction is exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
, and the mixture can reach the boiling point, if external cooling is not applied. The resulting product, diethyl 3,5-dimethylpyrrole-2,4-dicarboxylate, has been called Knorr's Pyrrole ever since. In the Scheme above, R2 = COOEt, and R1 = R3 = Me represent this original reaction.
Knorr's pyrrole can be derivatized in a number of useful manners. One equivalent of sodium hydroxide will saponify the 2-ester selectively. Dissolving Knorr's Pyrrole in concentrated sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
, and then pouring the resulting solution into water will hydrolyze the 4-ester group selectively. The 5-methyl group can be variously oxidized to chloromethyl, aldehyde, or carboxylic acid functionality by the use of stoichiometric sulfuryl chloride
Sulfuryl chloride
Sulfuryl chloride is an inorganic compound with the formula SO2Cl2. At room temperature, it is a colorless liquid with a pungent odor. Sulfuryl chloride is not found in nature, as can be inferred from its rapid hydrolysis....
in glacial acetic acid. Alternatively, the nitrogen atom can be alkylated. The two ester positions can be more smoothly differentiated by incorporating benzyl
Benzyl
In organic chemistry, benzyl is the term used to describe the substituent or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene ring attached to a CH2 group.-Nomenclature:...
or tertiary-butyl groups via the corresponding acetoacetate esters. Benzyl groups can be removed by catalytic hydrogenolysis
Hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes "lysis" by hydrogen. The heteroatom may vary, but it usually is oxygen, nitrogen, or sulfur. A related reaction is hydrogenation, where hydrogen is added to the molecule, without...
over palladium on carbon
Palladium on carbon
Palladium on carbon, often referred to as Pd/C, is a form of palladium used for catalysis. It is usually used for catalytic hydrogenations in organic chemistry...
, and tertiary-butyl groups can be removed by treatment with trifluoroacetic acid
Trifluoroacetic acid
Trifluoroacetic acid is the simplest stable perfluorinated carboxylic acid chemical compound, with the formula CF3CO2H. It is a strong carboxylic acid due to the influence of the electronegative trifluoromethyl group. TFA is almost 100,000-fold more acidic than acetic acid...
, or boiling aqueous acetic acid. R1 and R3 (as well as R2 and "Et") can be varied by the application of appropriate beta-ketoesters readily made by a synthesis emanating from acid chlorides, Meldrum's acid
Meldrum's acid
Meldrum's acid or 2,2-dimethyl-1,3-dioxane-4,6-dione is an organic compound. The compound was first made in 1908 by Andrew Norman Meldrum by a condensation reaction of malonic acid with acetone in acetic anhydride and sulfuric acid. Meldrum misidentified the structure as a β-lactone of...
, and the alcohol of one's choice. Ethyl and benzyl esters are easily made thereby, and the reaction is noteworthy in that even the highly hindered tertiary-butyl alcohol gives very high yields in this synthesis.
Levi and Zanetti extended the Knorr synthesis in 1894 to the use of acetylacetone
Acetylacetone
Acetylacetone is an organic compound that famously exists in two tautomeric forms that rapidly interconvert. The less stable tautomer is a diketone formally named pentane-2,4-dione. The more common tautomer is the enol form. The pair of tautomers rapidly interconvert and are treated as a single...
(2,4-pentanedione) in reaction with ethyl 2-oximinoacetoacetate. The result was ethyl 4-acetyl-3,5-dimethylpyrrole-2-carboxylate, where "OEt" = R1 = R3 = Me, and R2 = COOEt. The 4-acetyl group could easily be reduced to a 4-ethyl group by use of the Wolff-Kishner reduction
Wolff-Kishner reduction
The Wolff–Kishner reduction is a chemical reaction that fully reduces a ketone to an alkane.The method originally involved heating the hydrazine with sodium ethoxide in a sealed vessel at about 180 °C. Other bases have been found equally effective...
(hydrazine and alkali, heated); hydrogenolysis, or the use of diborane
Diborane
Diborane is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless gas at room temperature with a repulsively sweet odor. Diborane mixes well with air, easily forming explosive mixtures. Diborane will ignite spontaneously in moist air at room temperature...
. Benzyl or tertiary-butyl acetoacetates also work well in this system, and with close temperature control, the tertiary-butyl system gives a very high yield (close to 80%). N,N-dialkyl pyrrole-2- and/or 4-carboxamides may be prepared by the use of N,N-dialkyl acetoacetamides in the synthesis. Even thioesters have been successfully prepared, using the method. As for the nitrosation of beta-ketoesters, despite the numerous literature specifications of tight temperature control on the nitrosation, the reaction behaves almost like a titration, and the mixture can be allowed to reach even 40 degrees Celsius without significantly impacting the final yield.
Related synthesis
There are a number of important syntheses of pyrroles that are operated in the manner of the Knorr Synthesis, despite having mechanisms of very different connectivity between the starting materials and the pyrrolic product.Fischer and Fink found that Zanetti's synthesis from 2,4-pentanedione and ethyl 2-oximinoacetoacetate gave ethyl 3,5-dimethylpyrrole-2-carboxylate as a trace byproduct. Similarly, 3-ketobutyraldehyde diethyl acetal led to the formation of ethyl 5-methylpyrrole-2-carboxylate. Both of these products resulted from the loss of the acetyl group from the inferred ethyl 2-aminoacetoacetate intermediate. An important product of the Fischer-Fink synthesis was ethyl 4,5-dimethylpyrrole-2-carboxylate, made from ethyl 2-oximinoacetoacetate and 2-methyl-3-oxobutanal, in turn made by the Claisen condensation
Claisen condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone...
of 2-butanone with ethyl formate
Ethyl formate
Ethyl formate is an ester formed when ethanol reacts with formic acid . It is also known as ethyl methanoate because formic acid is also known as methanoic acid. Ethyl formate has the characteristic smell of rum and is also partially responsible for the flavor of raspberries.-Exposure:Ethyl...
.,
G.G. Kleinspehn reported that the Fischer-Fink connectivity could be forced to occur exclusively, by the use of diethyl oximinomalonate in the synthesis, with 2,4-pentanedione, or its 3-alkyl substituted derivatives. Yields were high, around 60%, and this synthesis eventually came to be one of the most important in the repertory. Yields were significantly improved, by the use of preformed diethyl aminomalonate (prepared by the hydrogenolysis of diethyl oximinomalonate in ethanol, over Pd/C), and adding a mixture of diethyl aminomalonate and the beta-diketone to actively boiling glacial acetic acid.
Meanwhile, Johnson had extended the Fischer-Fink synthesis by reacting 2-oximinoacetoacetate esters (ethyl, benzyl, or tertiary-butyl), with 3-alkyl substituted 2,4-pentanediones. Others extended the Kleinspehn synthesis by the use of unsymmetrical beta-diketones (such as 3-alkyl substituted 2,4-hexanediones), which preferentially reacted initially at the less hindered acetyl group and afforded the corresponding 5-methylpyrrole-2-carboxylate esters. N,N-Dialkyl 2-oximinoacetoacetamides also were found to give pyrroles when reacted under Knorr conditions with 3-substituted-2,4-pentanediones, in yields comparable to the corresponding esters (around 45%). However, when unsymmetrical diketones were used, it was found that the acetyl group from the acetoacetamide was retained in the product, and one of the acyl groups from the diketone had been lost. This same mechanism occurs to a minor extent in the acetoacetate ester systems, and had previously been detected radiochemically by Harbuck and Rapoport. Most of the above-described syntheses have application in the synthesis of porphyrins, bile pigments, and dipyrrins.

