
Isotope geochemistry
Encyclopedia
Isotope geochemistry is an aspect of geology
based upon study of the relative and absolute concentrations of the elements
and their isotope
s in the Earth
. Variations in the abundance of these isotopes, typically measured with an isotope ratio mass spectrometer or an accelerator mass spectrometer
, can reveal information about the age of a rock or the source of air or water. Isotope ratios can even shed light on chemical processes in the atmosphere. Broadly, the field of isotope geochemistry is divided into two branches: stable
and radiogenic isotope geochemistry.
and equilibrium fractionation
is very small; for this reason, enrichments are typically reported in "per mil" (‰, parts per thousand). These enrichments (δ) represent the ratio of heavy isotope to light isotope in the sample over the ratio of a standard. That is,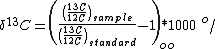
has two stable isotope
s, 12C and 13C, and one radioactive isotope, 14C
. Carbon isotope ratios are measured against Vienna Pee Dee Belemnite (VPDB). They have been used to track ocean circulation, among other things.
Carbon stable isotopes are fractionated primarily by photosynthesis
(Faure, 2004). The 13C/12C ratio is also an indicator of paleoclimate: a change in the ratio in the remains of plants indicates a change in the amount of photosynthetic activity, and thus in how favorable the environment was for the plants.
During photosynthesis, organisms using the C3 pathway show different enrichments compared to those using the C4 pathway, allowing scientists not only to distinguish organic matter from abiotic carbon, but also what type of photosynthetic pathway the organic matter was using.
has two stable isotopes, 14N, and 15N. The ratio between these is measured relative to nitrogen in ambient air. Nitrogen ratios are frequently linked to agricultural activities. Nitrogen isotope data has also been used to measure the amount of exchange of air between the stratosphere
and troposphere
using data from the greenhouse gas N2O
.
has three stable isotopes, 16O, 17O, and 18O. Oxygen ratios are measured relative to Vienna Standard Mean Ocean Water (VSMOW) or Vienna Pee Dee Belemnite (VPDB). Variations in oxygen isotope ratios are used to track both water movement, paleoclimate, and atmospheric gases such as ozone
and carbon dioxide
. Typically, the VPDB oxygen reference is used for paleoclimate, while VSMOW is used for most other applications. Oxygen isotopes appear in anomalous ratios in atmospheric ozone, resulting from mass-independent fractionation
. Isotope ratios in fossilized foraminifera
have been used to deduce the temperature of ancient seas.
has four stable isotopes, with the following abundances: 32S (0.9502), 33S (0.0075), 34S (0.0421) and 36S (0.0002). These abundances are compared to those found in Cañon Diablo troilite. Variations in sulfur isotope ratios are used to study the origin of sulfur in an ore
body and the temperature of formation of sulfur–bearing minerals.
has four stable isotopes - 204Pb, 206Pb, 207Pb, 208Pb and one common radioactive isotope 202Pb with a half-life
of ~53,000 years.
Lead is created in the Earth via decay of transuranic elements
, primarily uranium
and thorium
.
Lead isotope geochemistry
is useful for providing isotopic dates
on a variety of materials. Because the lead isotopes are created by decay of different transuranic elements, the ratios of the four lead isotopes to one another can be very useful in tracking the source of melts in igneous rocks, the source of sediments and even the origin of people via isotopic fingerprinting of their teeth, skin and bones.
It has been used to date ice core
s from the Arctic shelf, and provides information on the source of atmospheric lead pollution
.
Lead-lead isotopes has been successfully used in forensic science to fingerprint bullets, because each batch of ammunition has its own peculiar 204Pb/206Pb vs 207Pb/208Pb ratio.
-neodymium
is an isotope system which can be utilised to provide a date as well as isotopic fingerprints of geological materials, and various other materials including archaeological finds (pots, ceramics).
147Sm decays to produce 143Nd with a half life of 1.06x1011 years.
Dating is achieved usually by trying to produce an isochron
of several minerals within a rock specimen. The initial 143Nd/144Nd ratio is determined.
This initial ratio is modelled relative to CHUR - the Chondritic Uniform Reservoir - which is an approximation of the chondritic material which formed the solar system. CHUR was determined by analysing chondrite
and achondrite
meteorites.
The difference in the ratio of the sample relative to CHUR can give information on a model age of extraction from the mantle (for which an assumed evolution has been calculated relative to CHUR) and to whether this was extracted from a granitic source (depleted in radiogenic Nd), the mantle, or an enriched source.
and osmium
are siderophile elements which are present at very low abundances in the crust. Rhenium undergoes radioactive decay
to produce osmium. The ratio of non-radiogenic osmium to radiogenic osmium throughout time varies.
Rhenium prefers to enter sulfide
s more readily than osmium. Hence, during melting of the mantle, rhenium is stripped out, and prevents the osmium-osmium ratio from changing appreciably. This locks in an initial osmium ratio of the sample at the time of the melting event. Osmium-osmium initial ratios are used to determine the source characteristic and age of mantle melting events.
Protactinium
Uranium
is well mixed in the ocean, and its decay produces 231Pa and 230Th at a constant activity ratio (0.093). The decay products are rapidly removed by adsorption
on settling particles, but not at equal rates. 231Pa has a residence equivalent to the residence time of deep water
in the Atlantic basin (around 1000 yrs) but 230Th is removed more rapidly (centuries). Thermohaline circulation
effectively exports 231Pa from the Atlantic into the Southern Ocean
, while most of the 230Th remains in Atlantic sediments. As a result, there is a relationship between 231Pa/230Th in Atlantic sediments and the rate of overturning: faster overturning produces lower sediment 231Pa/230Th ratio, while slower overturning increases this ratio. The combination of δ13C and 231Pa/230Th can therefore provide a more complete insight into past circulation changes.
was trapped in the planet when it was created. Some 3He is being added by meteoric dust, primarily collecting on the bottom of oceans (although due to subduction
, all oceanic tectonic plates
are younger than continental plates). However, 3He will be degassed from oceanic sediment during subduction
, so cosmogenic 3He is not affecting the concentration or noble gas
ratios of the mantle
.
Helium-3 is created by cosmic ray
bombardment, and by lithium
spallation reactions which generally occur in the crust. Lithium spallation
is the process by which a high-energy neutron bombards a lithium
atom, creating a 3He and a 4He ion. This requires significant lithium to adversely affect the 3He/4He ratio.
All degassed helium is lost to space eventually, due to the average speed of helium exceeding the escape velocity
for the Earth. Thus, it is assumed the helium content and ratios of Earth's atmosphere
have remained essentially stable.
It has been observed that 3He is present in volcano
emissions and oceanic ridge samples. How 3He is stored in the planet is under investigation, but it is associated with the mantle
and is used as a marker of material of deep origin.
Due to similarities in helium
and carbon
in magma
chemistry, outgassing of helium requires the loss of volatile components
(water
, carbon dioxide
) from the mantle, which happens at depths of less than 60 km. However, 3He is transported to the surface primarily trapped in the crystal
lattice of minerals within fluid inclusions
.
Helium-4 is created by radiogenic production (by decay of uranium
/thorium
-series elements
). The continental crust
has become enriched with those elements relative to the mantle and thus more He4 is produced in the crust than in the mantle.
The ratio (R) of 3He to 4He is often used to represent 3He content. R usually is given as a multiple of the present atmospheric ratio (Ra).
Common values for R/Ra:
3He/4He isotope chemistry is being used to date groundwater
s, estimate groundwater flow rates, track water pollution, and provide insights into hydrothermal processes, igneous geology
and ore genesis
.
was released to the atmosphere during atmospheric testing of nuclear bombs. Radioactive decay of tritium produces the noble gas helium-3
. Comparing the ratio of tritium to helium-3 (3H/3He) allows estimation of the age of recent ground waters.
Geology
Geology is the science comprising the study of solid Earth, the rocks of which it is composed, and the processes by which it evolves. Geology gives insight into the history of the Earth, as it provides the primary evidence for plate tectonics, the evolutionary history of life, and past climates...
based upon study of the relative and absolute concentrations of the elements
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
and their isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s in the Earth
Earth
Earth is the third planet from the Sun, and the densest and fifth-largest of the eight planets in the Solar System. It is also the largest of the Solar System's four terrestrial planets...
. Variations in the abundance of these isotopes, typically measured with an isotope ratio mass spectrometer or an accelerator mass spectrometer
Accelerator mass spectrometry
Accelerator mass spectrometry differs from other forms of mass spectrometry in that it accelerates ions to extraordinarily high kinetic energies before mass analysis. The special strength of AMS among the mass spectrometric methods is its power to separate a rare isotope from an abundant...
, can reveal information about the age of a rock or the source of air or water. Isotope ratios can even shed light on chemical processes in the atmosphere. Broadly, the field of isotope geochemistry is divided into two branches: stable
Stable isotope
Stable isotopes are chemical isotopes that may or may not be radioactive, but if radioactive, have half-lives too long to be measured.Only 90 nuclides from the first 40 elements are energetically stable to any kind of decay save proton decay, in theory...
and radiogenic isotope geochemistry.
Stable isotope geochemistry
For most stable isotopes, the magnitude of fractionation from kineticKinetic fractionation
Kinetic fractionation is a process that separates stable isotopes from each other by their mass during unidirectional processes.One naturally occurring example of kinetic fractionation is the evaporation of seawater to form clouds...
and equilibrium fractionation
Equilibrium fractionation
Equilibrium isotope fractionation is the partial separation of isotopes between two or more substances in chemical equilibrium. Equilibrium fractionation is strongest at low temperatures, and forms the basis of the most widely used isotopic paleothermometers : D/H and 18O/16O records from ice...
is very small; for this reason, enrichments are typically reported in "per mil" (‰, parts per thousand). These enrichments (δ) represent the ratio of heavy isotope to light isotope in the sample over the ratio of a standard. That is,
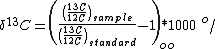
Carbon
CarbonCarbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
has two stable isotope
Stable isotope
Stable isotopes are chemical isotopes that may or may not be radioactive, but if radioactive, have half-lives too long to be measured.Only 90 nuclides from the first 40 elements are energetically stable to any kind of decay save proton decay, in theory...
s, 12C and 13C, and one radioactive isotope, 14C
Carbon-14
Carbon-14, 14C, or radiocarbon, is a radioactive isotope of carbon with a nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues , to date archaeological, geological, and hydrogeological...
. Carbon isotope ratios are measured against Vienna Pee Dee Belemnite (VPDB). They have been used to track ocean circulation, among other things.
Carbon stable isotopes are fractionated primarily by photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
(Faure, 2004). The 13C/12C ratio is also an indicator of paleoclimate: a change in the ratio in the remains of plants indicates a change in the amount of photosynthetic activity, and thus in how favorable the environment was for the plants.
During photosynthesis, organisms using the C3 pathway show different enrichments compared to those using the C4 pathway, allowing scientists not only to distinguish organic matter from abiotic carbon, but also what type of photosynthetic pathway the organic matter was using.
Nitrogen
NitrogenNitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
has two stable isotopes, 14N, and 15N. The ratio between these is measured relative to nitrogen in ambient air. Nitrogen ratios are frequently linked to agricultural activities. Nitrogen isotope data has also been used to measure the amount of exchange of air between the stratosphere
Stratosphere
The stratosphere is the second major layer of Earth's atmosphere, just above the troposphere, and below the mesosphere. It is stratified in temperature, with warmer layers higher up and cooler layers farther down. This is in contrast to the troposphere near the Earth's surface, which is cooler...
and troposphere
Troposphere
The troposphere is the lowest portion of Earth's atmosphere. It contains approximately 80% of the atmosphere's mass and 99% of its water vapor and aerosols....
using data from the greenhouse gas N2O
Nitrous oxide
Nitrous oxide, commonly known as laughing gas or sweet air, is a chemical compound with the formula . It is an oxide of nitrogen. At room temperature, it is a colorless non-flammable gas, with a slightly sweet odor and taste. It is used in surgery and dentistry for its anesthetic and analgesic...
.
Oxygen
OxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
has three stable isotopes, 16O, 17O, and 18O. Oxygen ratios are measured relative to Vienna Standard Mean Ocean Water (VSMOW) or Vienna Pee Dee Belemnite (VPDB). Variations in oxygen isotope ratios are used to track both water movement, paleoclimate, and atmospheric gases such as ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
and carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
. Typically, the VPDB oxygen reference is used for paleoclimate, while VSMOW is used for most other applications. Oxygen isotopes appear in anomalous ratios in atmospheric ozone, resulting from mass-independent fractionation
Mass-independent fractionation
Mass-independent fractionation refers to any chemical or physical process that acts to separate isotopes, where the amount of separation does not scale in proportion with the difference in the masses of the isotopes...
. Isotope ratios in fossilized foraminifera
Foraminifera
The Foraminifera , or forams for short, are a large group of amoeboid protists which are among the commonest plankton species. They have reticulating pseudopods, fine strands of cytoplasm that branch and merge to form a dynamic net...
have been used to deduce the temperature of ancient seas.
Sulfur
SulfurSulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
has four stable isotopes, with the following abundances: 32S (0.9502), 33S (0.0075), 34S (0.0421) and 36S (0.0002). These abundances are compared to those found in Cañon Diablo troilite. Variations in sulfur isotope ratios are used to study the origin of sulfur in an ore
Ore
An ore is a type of rock that contains minerals with important elements including metals. The ores are extracted through mining; these are then refined to extract the valuable element....
body and the temperature of formation of sulfur–bearing minerals.
Lead-lead isotope geochemistry
LeadLead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
has four stable isotopes - 204Pb, 206Pb, 207Pb, 208Pb and one common radioactive isotope 202Pb with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of ~53,000 years.
Lead is created in the Earth via decay of transuranic elements
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
, primarily uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
and thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
.
Lead isotope geochemistry
Geochemistry
The field of geochemistry involves study of the chemical composition of the Earth and other planets, chemical processes and reactions that govern the composition of rocks, water, and soils, and the cycles of matter and energy that transport the Earth's chemical components in time and space, and...
is useful for providing isotopic dates
Radiometric dating
Radiometric dating is a technique used to date materials such as rocks, usually based on a comparison between the observed abundance of a naturally occurring radioactive isotope and its decay products, using known decay rates...
on a variety of materials. Because the lead isotopes are created by decay of different transuranic elements, the ratios of the four lead isotopes to one another can be very useful in tracking the source of melts in igneous rocks, the source of sediments and even the origin of people via isotopic fingerprinting of their teeth, skin and bones.
It has been used to date ice core
Ice core
An ice core is a core sample that is typically removed from an ice sheet, most commonly from the polar ice caps of Antarctica, Greenland or from high mountain glaciers elsewhere. As the ice forms from the incremental build up of annual layers of snow, lower layers are older than upper, and an ice...
s from the Arctic shelf, and provides information on the source of atmospheric lead pollution
Air pollution
Air pollution is the introduction of chemicals, particulate matter, or biological materials that cause harm or discomfort to humans or other living organisms, or cause damage to the natural environment or built environment, into the atmosphere....
.
Lead-lead isotopes has been successfully used in forensic science to fingerprint bullets, because each batch of ammunition has its own peculiar 204Pb/206Pb vs 207Pb/208Pb ratio.
Samarium-neodymium
SamariumSamarium
Samarium is a chemical element with the symbol Sm, atomic number 62 and atomic weight 150.36. It is a moderately hard silvery metal which readily oxidizes in air. Being a typical member of the lanthanide series, samarium usually assumes the oxidation state +3...
-neodymium
Neodymium
Neodymium is a chemical element with the symbol Nd and atomic number 60. It is a soft silvery metal that tarnishes in air. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach. It is present in significant quantities in the ore minerals monazite and bastnäsite...
is an isotope system which can be utilised to provide a date as well as isotopic fingerprints of geological materials, and various other materials including archaeological finds (pots, ceramics).
147Sm decays to produce 143Nd with a half life of 1.06x1011 years.
Dating is achieved usually by trying to produce an isochron
Isochron dating
Isochron dating is a common technique of radiometric dating and is applied to date certain events, such as crystallization, metamorphism, shock events, and differentiation of precursor melts, in the history of rocks...
of several minerals within a rock specimen. The initial 143Nd/144Nd ratio is determined.
This initial ratio is modelled relative to CHUR - the Chondritic Uniform Reservoir - which is an approximation of the chondritic material which formed the solar system. CHUR was determined by analysing chondrite
Chondrite
Chondrites are stony meteorites that have not been modified due to melting or differentiation of the parent body. They formed when various types of dust and small grains that were present in the early solar system accreted to form primitive asteroids...
and achondrite
Achondrite
An achondrite is a stony meteorite that does not contain chondrules. It consists of material similar to terrestrial basalts or plutonic rocks and has been differentiated and reprocessed to a lesser or greater degree due to melting and recrystallization on or within meteorite parent bodies...
meteorites.
The difference in the ratio of the sample relative to CHUR can give information on a model age of extraction from the mantle (for which an assumed evolution has been calculated relative to CHUR) and to whether this was extracted from a granitic source (depleted in radiogenic Nd), the mantle, or an enriched source.
Rhenium-osmium
RheniumRhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-white, heavy, third-row transition metal in group 7 of the periodic table. With an average concentration of 1 part per billion , rhenium is one of the rarest elements in the Earth's crust. The free element has...
and osmium
Osmium
Osmium is a chemical element with the symbol Os and atomic number 76. Osmium is a hard, brittle, blue-gray or blue-blacktransition metal in the platinum family, and is the densest natural element. Osmium is twice as dense as lead. The density of osmium is , slightly greater than that of iridium,...
are siderophile elements which are present at very low abundances in the crust. Rhenium undergoes radioactive decay
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
to produce osmium. The ratio of non-radiogenic osmium to radiogenic osmium throughout time varies.
Rhenium prefers to enter sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
s more readily than osmium. Hence, during melting of the mantle, rhenium is stripped out, and prevents the osmium-osmium ratio from changing appreciably. This locks in an initial osmium ratio of the sample at the time of the melting event. Osmium-osmium initial ratios are used to determine the source characteristic and age of mantle melting events.
ProtactiniumProtactiniumProtactinium is a chemical element with the symbol Pa and atomic number 91. It is a dense, silvery-gray metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds where protactinium is usually present in the oxidation state +5, but can also assume...
:ThoriumThoriumThorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
Ratios - 231Pa / 230Th
UraniumUranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
is well mixed in the ocean, and its decay produces 231Pa and 230Th at a constant activity ratio (0.093). The decay products are rapidly removed by adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
on settling particles, but not at equal rates. 231Pa has a residence equivalent to the residence time of deep water
Deep Water
Deep Water is a suspense novel by Patricia Highsmith, first published in 1957 by Harper & Row.-Synopsis:In the small town of Little Wesley, intellectual publisher Victor Van Allen decides to discourage his wife Melinda’s many lovers by hinting to them that he may have killed her previous beau,...
in the Atlantic basin (around 1000 yrs) but 230Th is removed more rapidly (centuries). Thermohaline circulation
Thermohaline circulation
The term thermohaline circulation refers to a part of the large-scale ocean circulation that is driven by global density gradients created by surface heat and freshwater fluxes....
effectively exports 231Pa from the Atlantic into the Southern Ocean
Southern Ocean
The Southern Ocean comprises the southernmost waters of the World Ocean, generally taken to be south of 60°S latitude and encircling Antarctica. It is usually regarded as the fourth-largest of the five principal oceanic divisions...
, while most of the 230Th remains in Atlantic sediments. As a result, there is a relationship between 231Pa/230Th in Atlantic sediments and the rate of overturning: faster overturning produces lower sediment 231Pa/230Th ratio, while slower overturning increases this ratio. The combination of δ13C and 231Pa/230Th can therefore provide a more complete insight into past circulation changes.
Helium-3
Helium-3Helium-3
Helium-3 is a light, non-radioactive isotope of helium with two protons and one neutron. It is rare on Earth, and is sought for use in nuclear fusion research...
was trapped in the planet when it was created. Some 3He is being added by meteoric dust, primarily collecting on the bottom of oceans (although due to subduction
Subduction
In geology, subduction is the process that takes place at convergent boundaries by which one tectonic plate moves under another tectonic plate, sinking into the Earth's mantle, as the plates converge. These 3D regions of mantle downwellings are known as "Subduction Zones"...
, all oceanic tectonic plates
Tectonic Plates
Tectonic Plates is a 1992 independent Canadian film directed by Peter Mettler. Mettler also wrote the screenplay based on the play by Robert Lepage. The film stars Marie Gignac, Céline Bonnier and Robert Lepage.-Plot summary:...
are younger than continental plates). However, 3He will be degassed from oceanic sediment during subduction
Subduction
In geology, subduction is the process that takes place at convergent boundaries by which one tectonic plate moves under another tectonic plate, sinking into the Earth's mantle, as the plates converge. These 3D regions of mantle downwellings are known as "Subduction Zones"...
, so cosmogenic 3He is not affecting the concentration or noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
ratios of the mantle
Mantle (geology)
The mantle is a part of a terrestrial planet or other rocky body large enough to have differentiation by density. The interior of the Earth, similar to the other terrestrial planets, is chemically divided into layers. The mantle is a highly viscous layer between the crust and the outer core....
.
Helium-3 is created by cosmic ray
Cosmic ray
Cosmic rays are energetic charged subatomic particles, originating from outer space. They may produce secondary particles that penetrate the Earth's atmosphere and surface. The term ray is historical as cosmic rays were thought to be electromagnetic radiation...
bombardment, and by lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
spallation reactions which generally occur in the crust. Lithium spallation
Spallation
In general, spallation is a process in which fragments of material are ejected from a body due to impact or stress. In the context of impact mechanics it describes ejection or vaporization of material from a target during impact by a projectile...
is the process by which a high-energy neutron bombards a lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
atom, creating a 3He and a 4He ion. This requires significant lithium to adversely affect the 3He/4He ratio.
All degassed helium is lost to space eventually, due to the average speed of helium exceeding the escape velocity
Escape velocity
In physics, escape velocity is the speed at which the kinetic energy plus the gravitational potential energy of an object is zero gravitational potential energy is negative since gravity is an attractive force and the potential is defined to be zero at infinity...
for the Earth. Thus, it is assumed the helium content and ratios of Earth's atmosphere
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
have remained essentially stable.
It has been observed that 3He is present in volcano
Volcano
2. Bedrock3. Conduit 4. Base5. Sill6. Dike7. Layers of ash emitted by the volcano8. Flank| 9. Layers of lava emitted by the volcano10. Throat11. Parasitic cone12. Lava flow13. Vent14. Crater15...
emissions and oceanic ridge samples. How 3He is stored in the planet is under investigation, but it is associated with the mantle
Mantle (geology)
The mantle is a part of a terrestrial planet or other rocky body large enough to have differentiation by density. The interior of the Earth, similar to the other terrestrial planets, is chemically divided into layers. The mantle is a highly viscous layer between the crust and the outer core....
and is used as a marker of material of deep origin.
Due to similarities in helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
and carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
in magma
Magma
Magma is a mixture of molten rock, volatiles and solids that is found beneath the surface of the Earth, and is expected to exist on other terrestrial planets. Besides molten rock, magma may also contain suspended crystals and dissolved gas and sometimes also gas bubbles. Magma often collects in...
chemistry, outgassing of helium requires the loss of volatile components
Volatiles
In planetary science, volatiles are that group of chemical elements and chemical compounds with low boiling points that are associated with a planet's or moon's crust and/or atmosphere. Examples include nitrogen, water, carbon dioxide, ammonia, hydrogen, and methane, all compounds of C, H, O...
(water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
) from the mantle, which happens at depths of less than 60 km. However, 3He is transported to the surface primarily trapped in the crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
lattice of minerals within fluid inclusions
Fluid inclusions
thumb|250px|Trapped in a time capsule the same size as the diameter of a human hair, the ore-forming liquid in this inclusion was so hot and contained so much dissolved solids that when it cooled, crystals of halite, sylvite, gypsum, and hematite formed. As the samples cooled, the fluid shrank more...
.
Helium-4 is created by radiogenic production (by decay of uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
/thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
-series elements
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
). The continental crust
Continental crust
The continental crust is the layer of igneous, sedimentary, and metamorphic rocks which form the continents and the areas of shallow seabed close to their shores, known as continental shelves. This layer is sometimes called sial due to more felsic, or granitic, bulk composition, which lies in...
has become enriched with those elements relative to the mantle and thus more He4 is produced in the crust than in the mantle.
The ratio (R) of 3He to 4He is often used to represent 3He content. R usually is given as a multiple of the present atmospheric ratio (Ra).
Common values for R/Ra:
- Old continental crust: less than 1
- mid-ocean ridge basaltBasaltBasalt is a common extrusive volcanic rock. It is usually grey to black and fine-grained due to rapid cooling of lava at the surface of a planet. It may be porphyritic containing larger crystals in a fine matrix, or vesicular, or frothy scoria. Unweathered basalt is black or grey...
(MORB): 7 to 9 - Spreading ridge rocks: 9.1 plus or minus 3.6
- HotspotHotspot (geology)The places known as hotspots or hot spots in geology are volcanic regions thought to be fed by underlying mantle that is anomalously hot compared with the mantle elsewhere. They may be on, near to, or far from tectonic plate boundaries. There are two hypotheses to explain them...
rocks: 5 to 42 - Ocean and terrestrial water: 1
- Sedimentary formation water: less than 1
- Thermal spring water: 3 to 11
3He/4He isotope chemistry is being used to date groundwater
Groundwater
Groundwater is water located beneath the ground surface in soil pore spaces and in the fractures of rock formations. A unit of rock or an unconsolidated deposit is called an aquifer when it can yield a usable quantity of water. The depth at which soil pore spaces or fractures and voids in rock...
s, estimate groundwater flow rates, track water pollution, and provide insights into hydrothermal processes, igneous geology
Geology
Geology is the science comprising the study of solid Earth, the rocks of which it is composed, and the processes by which it evolves. Geology gives insight into the history of the Earth, as it provides the primary evidence for plate tectonics, the evolutionary history of life, and past climates...
and ore genesis
Ore genesis
The various theories of ore genesis explain how the various types of mineral deposits form within the Earth's crust. Ore genesis theories are very dependent on the mineral or commodity....
.
- (U-Th)/He dating of apatite as a thermal history tool
- USGS: Helium Discharge at Mammoth Mountain Fumarole (MMF)
Tritium/helium-3
TritiumTritium
Tritium is a radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of protium contains one proton and no neutrons...
was released to the atmosphere during atmospheric testing of nuclear bombs. Radioactive decay of tritium produces the noble gas helium-3
Helium-3
Helium-3 is a light, non-radioactive isotope of helium with two protons and one neutron. It is rare on Earth, and is sought for use in nuclear fusion research...
. Comparing the ratio of tritium to helium-3 (3H/3He) allows estimation of the age of recent ground waters.
See also
- Cosmogenic isotopes
- Environmental isotopesEnvironmental isotopesThe environmental isotopes are a subset of the isotopes, both stable and radioactive, which are the object of Isotope geochemistry.The most used environmental isotopes are:* deuterium* tritium* carbon-13* carbon-14* nitrogen-15* oxygen-18...
- GeochemistryGeochemistryThe field of geochemistry involves study of the chemical composition of the Earth and other planets, chemical processes and reactions that govern the composition of rocks, water, and soils, and the cycles of matter and energy that transport the Earth's chemical components in time and space, and...
- Isotopic signatureIsotopic signatureAn isotopic signature is a ratio of stable or unstable isotopes of particular elements found in an investigated material...
- Radiometric datingRadiometric datingRadiometric dating is a technique used to date materials such as rocks, usually based on a comparison between the observed abundance of a naturally occurring radioactive isotope and its decay products, using known decay rates...
General online stable isotope references
- Environmental Isotopes (University of OttawaUniversity of OttawaThe University of Ottawa is a bilingual, research-intensive, non-denominational, international university in Ottawa, Ontario. It is one of the oldest universities in Canada. It was originally established as the College of Bytown in 1848 by the Missionary Oblates of Mary Immaculate...
) - Fundamentals of Isotope Geochemistry (C. Kendall & E.A. Caldwell, chap.2 in Isotope Tracers in Catchment Hydrology [edited by C. Kendall & J.J. McDonnell], 1998)
- Stable Isotopes and Mineral Resource Investigations in the United States (USGS)
General
- Allègre C.J.Claude AllègreClaude Allègre is a French politician and scientist.- Scientific work :The main scientific area of Claude Allègre is geochemistry....
, 2008. Isotope Geology (Cambridge University PressCambridge University PressCambridge University Press is the publishing business of the University of Cambridge. Granted letters patent by Henry VIII in 1534, it is the world's oldest publishing house, and the second largest university press in the world...
). - Dickin A.P., 2005. Radiogenic Isotope Geology (Cambridge University PressCambridge University PressCambridge University Press is the publishing business of the University of Cambridge. Granted letters patent by Henry VIII in 1534, it is the world's oldest publishing house, and the second largest university press in the world...
). - Faure G., Mensing T.M. (2004), Isotopes: Principles and Applications (John Wiley & SonsJohn Wiley & SonsJohn Wiley & Sons, Inc., also referred to as Wiley, is a global publishing company that specializes in academic publishing and markets its products to professionals and consumers, students and instructors in higher education, and researchers and practitioners in scientific, technical, medical, and...
). - Hoefs J., 2004. Stable Isotope Geochemistry (Springer Verlag).
- Sharp Z., 2006. Principles of Stable Isotope Geochemistry (Prentice HallPrentice HallPrentice Hall is a major educational publisher. It is an imprint of Pearson Education, Inc., based in Upper Saddle River, New Jersey, USA. Prentice Hall publishes print and digital content for the 6-12 and higher-education market. Prentice Hall distributes its technical titles through the Safari...
).
3He/4He
- Burnard P.G., Farley K.A., & Turner G., 1998. "Multiple fluid pulses in a Samoan harzburgiteHarzburgiteThe ultramafic igneous rock, harzburgite, is a variety of peridotite consisting mostly of the two minerals, olivine and low-calcium pyroxene ; it is named for occurrences in the Harz Mountains of Germany. It commonly contains a few percent chromium-rich spinel as an accessory mineral...
", Chemical Geology, 147: 99-114. - Kirstein L. & Timmerman M., 2000. "Evidence of the proto-Iceland lume in northwestern Ireland at 42Ma from helium isotopes", Journal of the Geological Society, London, 157: 923-927.
- Porcelli D. & Halliday A.N., 2001. "The core as a possible source of mantle helium", Earth and Planetary Science Letters, 192: 45-56.
Re-Os
- Arne D., Bierlein F.P., Morgan J.W., & Stein H.J., 2001. "Re-Os dating of sulfides associated with gold mineralisation in central Victoria, Australia", Economic Geology, 96: 1455-1459.
- Martin C., 1991. "Osmium isotopic characteristics of mantle-derived rocks", Geochimica et Cosmochimica Acta, 55: 1421-1434.
External Links
- National Isotope Development Center Reference information on isotopes, and coordination and management of isotope production, availability, and distribution
- Isotope Development & Production for Research and Applications (IDPRA) U.S. Department of Energy program for isotope production and production research and development

