
Isotope analysis
Encyclopedia
Isotope analysis is the identification of isotopic signature
, the distribution of certain stable isotopes and chemical elements
within chemical compounds. This can be applied to a food web to make it possible to draw direct inferences regarding diet, trophic level
, and subsistence. Isotope ratios are measured using mass spectrometry
, which separates the different isotopes of an element on the basis of their mass-to-charge ratio
.
exists as three naturally occurring isotopes, of which the Relative Abundances are listed below:
Present in the ratios above, oxygen atoms of all isotopes are incorporated into molecules, including water. All isotopes of oxygen have similar properties, but water that incorporates 16O evaporates preferentially to water with the 18O isotope.
In isotopic analysis, the absolute abundances of isotopic oxygen are not considered. Rather, the ratio of 18O to 16O (or δ18O) in the sample is compared to the VSMOW
standard ratio using the equation: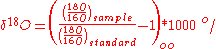
The values are reported in permil (‰). While the differences between samples and the standard may appear small, a difference of even 1 permil is significant.
Bone is continually remodelled throughout the lifetime of an individual. Although the rate of turnover of isotopic oxygen in hydroxyapatite is not fully known, it is assumed to be similar to that of collagen; approximately 10 years. Consequently, should an individual remain in a region for 10 years or longer, the isotopic oxygen ratios in the bone hydroxyapatite would reflect the oxygen ratios present in that region.
Teeth are not subject to continual remodelling and so their isotopic oxygen ratios remain constant from the time of formation. The isotopic oxygen ratios, then, of teeth represent the ratios of the region in which the individual was born and raised. Where deciduous teeth are present, it is also possible to determine the age at which a child was weaned. Breast milk production draws upon the body water of the mother, which has higher levels of 18O due to the preferential loss of 16O through sweat, urine, and expired water vapour.
While teeth are more resistant to chemical and physical changes over time, both are subject to post-depositional diagenesis. As such, isotopic analysis makes use of the more resistant phosphate groups, rather than the less abundant hydroxyl group or the more likely diagenetic carbonate groups present.
that may affect the original isotopic signal. Carbon
and nitrogen
isotope composition are used to reconstruct diet, and oxygen
isotopes are used to determine geographic origin. Strontium and lead isotopes in teeth and bone can sometimes be used to reconstruct migration in human populations and cultural affinity.
Carbon isotopes are taken up through the diet of animals during their lifetime, oxygen isotopes being taken up through the water they drink. Examining the 12C/13C isotope ratio, it is possible to determine whether animals ate predominantly C3
or C4
plants. This process ends with the organism's death, from this point on isotopes no longer accumulate in the body, but do undergo degradation. For best result the researcher would need to know the original levels, or an estimation thereof, of isotopes in the organism at the time of its death.
To obtain an accurate picture of palaeodiets, it is important to understand processes of diagenesis
that may affect the original isotopic signal. It is also important for the researcher to know the variations of isotopes within individuals, between individuals, and over time.
The main elements used in isotope ecology are carbon, nitrogen, oxygen, hydrogen and sulfur.
Analysis of the ratio of 18O to 16O in the shells
of the Colorado Delta clam was used to assess the historical extent of the estuary
in the Colorado River Delta
prior to construction of upstream dams.
s because they can help scientists in understanding source links and process information in marine food webs. These analysis can also be used to a certain degree in terrestrial systems. Certain isotopes can signify distinct primary producers forming the bases of food web
s and trophic level
positioning. The stable isotope compositions are expressed in terms of delta values (δ), which are parts per thousand (‰) differences from a standard. They express the number of isotopes that are in a sample. The values are expressed as:
δX = [(Rsample / Rstandard) – 1] x 103
where X represents the isotope of interest and R represents the ratio of the isotope of interest and its natural form (i.e. 13C/12C). Higher values indicate increases in the amount of heavy isotopes and lower values indicate decreases. The standard reference for carbon, nitrogen and sulfur are PeeDee Limestone, nitrogen gas in the atmosphere and Cañyon Diablo meteorite respectively. Analysis is usually done using a mass spectrometer, detecting small differences between gaseous elements. Analysis of a sample can cost anywhere from $30 to $100. Stable isotopes assist scientists in analyzing animal diets and food webs by examining the animal tissues that bear a fixed isotopic enrichment or depletion vs. the diet. Muscle or protein fractions have become the most common animal tissue used to examine the isotopes because they represent the assimilated nutrients in their diet. The main advantage to using stable isotope analysis as opposed to stomach content observations is that no matter what the status is of the animal's stomach (empty or not), the isotope tracers in the tissues will give us an understanding of its trophic position and food source. The three major isotopes used in aquatic ecosystem food web analysis are 13C, 15N and 34S. While all three indicate information on trophic dynamics, it is common to perform analysis on at least two of the previously mentioned 3 isotopes for better understanding of marine trophic interactions and for stronger results.
Carbon isotopes aid us in determining the primary production
source responsible for the energy flow in an ecosystem. The transfer of 13C through trophic levels remains relatively the same, except for a small increase (an enrichment < 1 ‰). Large differences of δ13C between animals indicate that they have different food sources or that their food webs are based on different primary producers (i.e. different species of phytoplankton, marsh grasses.) Because δ13C indicates the original source of primary producers, the isotopes can also help us determine shifts in diets, both short term, long term or permanent. These shifts may even correlate to seasonal changes, reflecting phytoplankton abundance. Scientists have found that there can be wide ranges of δ13C values in phytoplankton populations over a geographic region. While it is not quite certain as to why this may be, there are several hypotheses for this occurrence. These include isotopes within dissolved inorganic carbon pools (DIC) may vary with temperature and location and that growth rates of phytoplankton may affect their uptake of the isotopes. δ13C has been used in determining migration of juvenile animals from sheltered inshore areas to offshore locations by examining the changes in their diets. A study by Fry (1983) studied the isotopic compositions in juvenile shrimp of south Texas grass flats. Fry found that at the beginning of the study the shrimp had isotopic values of δ13C = -11 to -14‰ and 6-8‰ for δ15N and δ34S. As the shrimp matured and migrated offshore, the isotopic values changed to those resembling offshore organisms (δ13C= -15‰ and δ15N = 11.5‰ and δ34S = 16‰).
While there is no enrichment of 34S between trophic levels, the stable isotope can be useful in distinguishing benthic
vs. pelagic producers and marsh
vs. phytoplankton
producers. Similar to 13C, it can also help distinguish between different phytoplankton as the key primary producers in food webs. The differences between seawater sulfates and sulfides (~21‰ vs -10‰) aid scientists in the discriminations. Sulfur tends to be more plentiful in less aerobic areas, such as benthic systems and marsh plants, than the pelagic and more aerobic systems. Thus, in the benthic systems, there are smaller δ34S values.
Nitrogen isotopes indicate the trophic level position of various marine organisms (reflective of the time the tissue samples were taken). There is a larger enrichment component with δ15N because its retention is higher than that of 14N. This can be seen by analyzing the waste of organisms. Cattle urine has shown that there is a depletion of 15N relative to the diet. As organisms eat each other, the 15N isotopes are transferred to the predators. Thus, organisms higher in the trophic pyramid have accumulated higher levels of 15N ( and higher δ15N values) relative to their prey and others before them in the food web. Numerous studies on marine ecosystems have shown that on average there is a 3.2‰ enrichment of 15N vs. diet between different trophic level species in ecosystems In the Baltic sea, Hansson et al. (1997) found that when analyzing a variety of creatures (such as particulate
organic matter (phytoplankton), zooplankton
, mysids, sprat, smelt and herring,) there was an apparent fractionation of 2.4‰ between consumers and their apparent prey.
In addition to trophic positioning of organisms, δ15N values have become commonly used in distinguishing between land derived and natural sources of nutrients. As water travels from septic tanks to aquifers, the nitrogen rich water is delivered into coastal areas. Waste-water nitrate has higher concentrations of 15N than the nitrate that is found in natural soils in near shore zones. For bacteria, it is more convenient for them to uptake 14N as opposed to 15N because it is a lighter element and easier to metabolize. Thus, due to bacteria's preference when performing biogeochemical processes
such as denitrification
and volatilization of ammonia, 14N is removed from the water at a faster rate than 15N, resulting in more 15N entering the aquifer. 15N is roughly 10-20‰ as opposed to the natural 15N values of 2-8‰. The inorganic nitrogen that is emitted from septic tanks and other human-derived sewage is usually in the form of NH4+. Once the nitrogen enters the estuaries via groundwater, it is thought that because there is more 15N entering, that there will also be more 15N in the inorganic nitrogen pool delivered and that it is picked up more by producers taking up N. Even though 14N is easier to take up, because there is much more 15N, there will still be higher amounts assimilated than normal. These levels of δ15N can be examined in creatures that live in the area and are non migratory (such as macrophyte
s, clams and even some fish). This method of identifying high levels of nitrogen input is becoming a more and more popular method in attempting to monitor nutrient input into estuaries and coastal ecosystems. Environmental managers have become more and more concerned about measuring anthropogenic nutrient inputs into estuaries because excess in nutrients can lead to eutrophication
and hypoxic events
, eliminating organisms from an area entirely.
Isotope analysis can be used by forensic investigators to determine whether two or more samples of explosives are of a common origin. Most high explosives
contain carbon, hydrogen, nitrogen and oxygen atoms and thus comparing their relative abundances of isotopes can reveal the existence of a common origin. Researchers have also shown that analysis of the 12C/13C ratios can locate the country of origin for a given explosive.
Stable isotopic analysis has also been used in the identification of drug trafficking routes. Isotopic abundances are different in morphine grown from poppies in South-east Asia versus poppies grown in South-West Asia. The same is applied to cocaine that is derived from Bolivia and that from Columbia.
s, 16O is preferentially evaporated from the colder oceans, leaving the slightly heavier and more sluggish 18O behind. Organisms such as foraminifera which combine oxygen dissolved in the surrounding water with carbon and calcium to build their shells therefore incorporate the temperature-dependent 18O to 16O ratio. When these organisms die, they settle out on the sea bed, preserving a long and invaluable record of global climate change through much of the Quaternary
. Similarly, ice cores on land are enriched in the heavier 18O relative to 16O during warmer climatic phases (interglacial
s) as more energy is available for the evaporation of the heavier 18O isotope. The oxygen isotope record preserved in the ice cores is therefore a `mirror` of the record contained in ocean sediments.
Oxygen isotopes preserve a record of the effects of the Milankovitch cycles
on climate change during the Quaternary, revealing an approximately 100,000-year cyclicity in the Earth's climate.
Isotopic signature
An isotopic signature is a ratio of stable or unstable isotopes of particular elements found in an investigated material...
, the distribution of certain stable isotopes and chemical elements
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
within chemical compounds. This can be applied to a food web to make it possible to draw direct inferences regarding diet, trophic level
Trophic level
The trophic level of an organism is the position it occupies in a food chain. The word trophic derives from the Greek τροφή referring to food or feeding. A food chain represents a succession of organisms that eat another organism and are, in turn, eaten themselves. The number of steps an organism...
, and subsistence. Isotope ratios are measured using mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
, which separates the different isotopes of an element on the basis of their mass-to-charge ratio
Mass-to-charge ratio
The mass-to-charge ratio ratio is a physical quantity that is widely used in the electrodynamics of charged particles, e.g. in electron optics and ion optics. It appears in the scientific fields of lithography, electron microscopy, cathode ray tubes, accelerator physics, nuclear physics, Auger...
.
Oxygen isotopes
OxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
exists as three naturally occurring isotopes, of which the Relative Abundances are listed below:
- 16O = 99.763%
- 17OOxygen-17Oxygen-17 is a low abundant isotope of oxygen . Being the only stable isotope of oxygen possessing a nuclear spin and the unique characteristic of field-independent relaxation it enables NMR studies of metabolic pathways of compounds incorporating oxygen at high magnetic fields Oxygen-17 is a low...
= 0.0375% - 18OOxygen-18Oxygen-18 is a natural, stable isotope of oxygen and one of the environmental isotopes.18O is an important precursor for the production of fluorodeoxyglucose used in positron emission tomography...
= 0.1995%
Present in the ratios above, oxygen atoms of all isotopes are incorporated into molecules, including water. All isotopes of oxygen have similar properties, but water that incorporates 16O evaporates preferentially to water with the 18O isotope.
In isotopic analysis, the absolute abundances of isotopic oxygen are not considered. Rather, the ratio of 18O to 16O (or δ18O) in the sample is compared to the VSMOW
VSMOW
Vienna Standard Mean Ocean Water is a water standard defining the isotopic composition of water. It was promulgated by the International Atomic Energy Agency in 1968....
standard ratio using the equation:
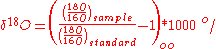
The values are reported in permil (‰). While the differences between samples and the standard may appear small, a difference of even 1 permil is significant.
Variation by latitude
As moist air masses are carried away from the equator by the prevailing weather patterns they lose the heavier, more easily condensed, 18O water leading to lower and lower isotopic oxygen ratios toward the poles. Consequently, the amount of 18O relative to 16O in the water vapor becomes less and less as it approaches the poles, preferentially losing 18O water in the form of rain and snow.Variation occurring from the hydrological cycle
The ratios of isotopic oxygen are also differentially affected by global weather patterns and regional topography as moisture is transported. Areas of lower humidity cause the preferential loss of 18O water in the form of vapor and precipitation. Furthermore, evaporated 16O water returns preferentially to the atmospheric system as it evaporates and 18O remains in liquid form or is incorporated into the body water of plants and animals.Tissues affected
Isotopic oxygen is incorporated into the body primarily through ingestion at which point it is used in the formation of, for archaeological purposes, bones and teeth. The oxygen is incorporated into the hydroxylcarbonic apatite of bone and tooth enamel.Bone is continually remodelled throughout the lifetime of an individual. Although the rate of turnover of isotopic oxygen in hydroxyapatite is not fully known, it is assumed to be similar to that of collagen; approximately 10 years. Consequently, should an individual remain in a region for 10 years or longer, the isotopic oxygen ratios in the bone hydroxyapatite would reflect the oxygen ratios present in that region.
Teeth are not subject to continual remodelling and so their isotopic oxygen ratios remain constant from the time of formation. The isotopic oxygen ratios, then, of teeth represent the ratios of the region in which the individual was born and raised. Where deciduous teeth are present, it is also possible to determine the age at which a child was weaned. Breast milk production draws upon the body water of the mother, which has higher levels of 18O due to the preferential loss of 16O through sweat, urine, and expired water vapour.
While teeth are more resistant to chemical and physical changes over time, both are subject to post-depositional diagenesis. As such, isotopic analysis makes use of the more resistant phosphate groups, rather than the less abundant hydroxyl group or the more likely diagenetic carbonate groups present.
Applications
Isotope analysis has widespread applicability in the natural sciences. These include numerous applications in the biological, earth and environmental sciences.Reconstructing palaeodiet
Bone recovered from archaeological sites can be analysed isotopically for information regarding diet and migration. Tooth enamel and soil surrounding or clinging to the remains may also be used in isotopic analysis. To obtain an accurate picture of palaeodiets, it is important to understand processes of diagenesisDiagenesis
In geology and oceanography, diagenesis is any chemical, physical, or biological change undergone by a sediment after its initial deposition and during and after its lithification, exclusive of surface alteration and metamorphism. These changes happen at relatively low temperatures and pressures...
that may affect the original isotopic signal. Carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
and nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
isotope composition are used to reconstruct diet, and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
isotopes are used to determine geographic origin. Strontium and lead isotopes in teeth and bone can sometimes be used to reconstruct migration in human populations and cultural affinity.
Carbon isotopes are taken up through the diet of animals during their lifetime, oxygen isotopes being taken up through the water they drink. Examining the 12C/13C isotope ratio, it is possible to determine whether animals ate predominantly C3
C3 carbon fixation
carbon fixation is a metabolic pathway for carbon fixation in photosynthesis. This process converts carbon dioxide and ribulose bisphosphate into 3-phosphoglycerate through the following reaction:...
or C4
C4 carbon fixation
C4 carbon fixation is one of three biochemical mechanisms, along with and CAM photosynthesis, used in carbon fixation. It is named for the 4-carbon molecule present in the first product of carbon fixation in these plants, in contrast to the 3-carbon molecule products in plants. fixation is an...
plants. This process ends with the organism's death, from this point on isotopes no longer accumulate in the body, but do undergo degradation. For best result the researcher would need to know the original levels, or an estimation thereof, of isotopes in the organism at the time of its death.
To obtain an accurate picture of palaeodiets, it is important to understand processes of diagenesis
Diagenesis
In geology and oceanography, diagenesis is any chemical, physical, or biological change undergone by a sediment after its initial deposition and during and after its lithification, exclusive of surface alteration and metamorphism. These changes happen at relatively low temperatures and pressures...
that may affect the original isotopic signal. It is also important for the researcher to know the variations of isotopes within individuals, between individuals, and over time.
Sourcing archaeological materials
Isotope analysis has been particularly useful in archaeology as a means of characterization. Characterization of artifacts involves determining the isotopic composition of possible source materials such as metal ore bodies and comparing these data to the isotopic composition of analyzed artifacts. A wide range of archaeological materials such as metals, glass and lead-based pigments have been sourced using isotopic characterization. Particularly in the Bronze Age Mediterranean Lead Isotope Analysis has been a useful tool for determining the sources of metals and an important indicator of trade patterns. Interpretation of Lead Isotope Data is, however, often contentious and faces numerous instrumental and methodological challenges. Problems such as the mixing and re-using of metals form different sources, limited reliable data and contamination of samples can be difficult problems in interpretation.Ecology
All biologically active elements exist in a number of different isotopic forms, of which two or more are stable. For example most carbon is present as 12C, with approximately 1% being 13C. The ratio of the two isotopes may be altered by biological and geophysical processes, and these differences can be utilized in a number of ways by ecologists.The main elements used in isotope ecology are carbon, nitrogen, oxygen, hydrogen and sulfur.
Analysis of the ratio of 18O to 16O in the shells
Seashell
A seashell or sea shell, also known simply as a shell, is a hard, protective outer layer created by an animal that lives in the sea. The shell is part of the body of the animal. Empty seashells are often found washed up on beaches by beachcombers...
of the Colorado Delta clam was used to assess the historical extent of the estuary
Estuary
An estuary is a partly enclosed coastal body of water with one or more rivers or streams flowing into it, and with a free connection to the open sea....
in the Colorado River Delta
Colorado River Delta
The Colorado River Delta is the region where the Colorado River flows into the Gulf of California . The delta is part of a larger geologic region called the Salton Trough. Historically, the interaction of the river’s flow and the ocean’s tide created a dynamic environment, supporting freshwater,...
prior to construction of upstream dams.
Stable isotope analysis in aquatic ecosystems
Stable isotopes have become a popular method for understanding aquatic ecosystemAquatic ecosystem
An aquatic ecosystem is an ecosystem in a body of water. Communities of organisms that are dependent on each other and on their environment live in aquatic ecosystems. The two main types of aquatic ecosystems are marine ecosystems and freshwater ecosystems....
s because they can help scientists in understanding source links and process information in marine food webs. These analysis can also be used to a certain degree in terrestrial systems. Certain isotopes can signify distinct primary producers forming the bases of food web
Food web
A food web depicts feeding connections in an ecological community. Ecologists can broadly lump all life forms into one of two categories called trophic levels: 1) the autotrophs, and 2) the heterotrophs...
s and trophic level
Trophic level
The trophic level of an organism is the position it occupies in a food chain. The word trophic derives from the Greek τροφή referring to food or feeding. A food chain represents a succession of organisms that eat another organism and are, in turn, eaten themselves. The number of steps an organism...
positioning. The stable isotope compositions are expressed in terms of delta values (δ), which are parts per thousand (‰) differences from a standard. They express the number of isotopes that are in a sample. The values are expressed as:
δX = [(Rsample / Rstandard) – 1] x 103
where X represents the isotope of interest and R represents the ratio of the isotope of interest and its natural form (i.e. 13C/12C). Higher values indicate increases in the amount of heavy isotopes and lower values indicate decreases. The standard reference for carbon, nitrogen and sulfur are PeeDee Limestone, nitrogen gas in the atmosphere and Cañyon Diablo meteorite respectively. Analysis is usually done using a mass spectrometer, detecting small differences between gaseous elements. Analysis of a sample can cost anywhere from $30 to $100. Stable isotopes assist scientists in analyzing animal diets and food webs by examining the animal tissues that bear a fixed isotopic enrichment or depletion vs. the diet. Muscle or protein fractions have become the most common animal tissue used to examine the isotopes because they represent the assimilated nutrients in their diet. The main advantage to using stable isotope analysis as opposed to stomach content observations is that no matter what the status is of the animal's stomach (empty or not), the isotope tracers in the tissues will give us an understanding of its trophic position and food source. The three major isotopes used in aquatic ecosystem food web analysis are 13C, 15N and 34S. While all three indicate information on trophic dynamics, it is common to perform analysis on at least two of the previously mentioned 3 isotopes for better understanding of marine trophic interactions and for stronger results.
13C
Carbon isotopes aid us in determining the primary production
Primary production
400px|thumb|Global oceanic and terrestrial photoautotroph abundance, from September [[1997]] to August 2000. As an estimate of autotroph biomass, it is only a rough indicator of primary production potential, and not an actual estimate of it...
source responsible for the energy flow in an ecosystem. The transfer of 13C through trophic levels remains relatively the same, except for a small increase (an enrichment < 1 ‰). Large differences of δ13C between animals indicate that they have different food sources or that their food webs are based on different primary producers (i.e. different species of phytoplankton, marsh grasses.) Because δ13C indicates the original source of primary producers, the isotopes can also help us determine shifts in diets, both short term, long term or permanent. These shifts may even correlate to seasonal changes, reflecting phytoplankton abundance. Scientists have found that there can be wide ranges of δ13C values in phytoplankton populations over a geographic region. While it is not quite certain as to why this may be, there are several hypotheses for this occurrence. These include isotopes within dissolved inorganic carbon pools (DIC) may vary with temperature and location and that growth rates of phytoplankton may affect their uptake of the isotopes. δ13C has been used in determining migration of juvenile animals from sheltered inshore areas to offshore locations by examining the changes in their diets. A study by Fry (1983) studied the isotopic compositions in juvenile shrimp of south Texas grass flats. Fry found that at the beginning of the study the shrimp had isotopic values of δ13C = -11 to -14‰ and 6-8‰ for δ15N and δ34S. As the shrimp matured and migrated offshore, the isotopic values changed to those resembling offshore organisms (δ13C= -15‰ and δ15N = 11.5‰ and δ34S = 16‰).
34S
While there is no enrichment of 34S between trophic levels, the stable isotope can be useful in distinguishing benthic
Benthos
Benthos is the community of organisms which live on, in, or near the seabed, also known as the benthic zone. This community lives in or near marine sedimentary environments, from tidal pools along the foreshore, out to the continental shelf, and then down to the abyssal depths.Many organisms...
vs. pelagic producers and marsh
Marsh
In geography, a marsh, or morass, is a type of wetland that is subject to frequent or continuous flood. Typically the water is shallow and features grasses, rushes, reeds, typhas, sedges, other herbaceous plants, and moss....
vs. phytoplankton
Phytoplankton
Phytoplankton are the autotrophic component of the plankton community. The name comes from the Greek words φυτόν , meaning "plant", and πλαγκτός , meaning "wanderer" or "drifter". Most phytoplankton are too small to be individually seen with the unaided eye...
producers. Similar to 13C, it can also help distinguish between different phytoplankton as the key primary producers in food webs. The differences between seawater sulfates and sulfides (~21‰ vs -10‰) aid scientists in the discriminations. Sulfur tends to be more plentiful in less aerobic areas, such as benthic systems and marsh plants, than the pelagic and more aerobic systems. Thus, in the benthic systems, there are smaller δ34S values.
15N
Nitrogen isotopes indicate the trophic level position of various marine organisms (reflective of the time the tissue samples were taken). There is a larger enrichment component with δ15N because its retention is higher than that of 14N. This can be seen by analyzing the waste of organisms. Cattle urine has shown that there is a depletion of 15N relative to the diet. As organisms eat each other, the 15N isotopes are transferred to the predators. Thus, organisms higher in the trophic pyramid have accumulated higher levels of 15N ( and higher δ15N values) relative to their prey and others before them in the food web. Numerous studies on marine ecosystems have shown that on average there is a 3.2‰ enrichment of 15N vs. diet between different trophic level species in ecosystems In the Baltic sea, Hansson et al. (1997) found that when analyzing a variety of creatures (such as particulate
Particle (ecology)
In marine and freshwater ecology, a particle is a small object. Particles can remain in suspension in the ocean or freshwater, however they eventually settle and accumulate as sediment. Some can enter the atmosphere through wave action where they can act as cloud condensation nuclei...
organic matter (phytoplankton), zooplankton
Zooplankton
Zooplankton are heterotrophic plankton. Plankton are organisms drifting in oceans, seas, and bodies of fresh water. The word "zooplankton" is derived from the Greek zoon , meaning "animal", and , meaning "wanderer" or "drifter"...
, mysids, sprat, smelt and herring,) there was an apparent fractionation of 2.4‰ between consumers and their apparent prey.
In addition to trophic positioning of organisms, δ15N values have become commonly used in distinguishing between land derived and natural sources of nutrients. As water travels from septic tanks to aquifers, the nitrogen rich water is delivered into coastal areas. Waste-water nitrate has higher concentrations of 15N than the nitrate that is found in natural soils in near shore zones. For bacteria, it is more convenient for them to uptake 14N as opposed to 15N because it is a lighter element and easier to metabolize. Thus, due to bacteria's preference when performing biogeochemical processes
Biogeochemical cycle
In ecology and Earth science, a biogeochemical cycle or substance turnover or cycling of substances is a pathway by which a chemical element or molecule moves through both biotic and abiotic compartments of Earth. A cycle is a series of change which comes back to the starting point and which can...
such as denitrification
Denitrification
Denitrification is a microbially facilitated process of nitrate reduction that may ultimately produce molecular nitrogen through a series of intermediate gaseous nitrogen oxide products....
and volatilization of ammonia, 14N is removed from the water at a faster rate than 15N, resulting in more 15N entering the aquifer. 15N is roughly 10-20‰ as opposed to the natural 15N values of 2-8‰. The inorganic nitrogen that is emitted from septic tanks and other human-derived sewage is usually in the form of NH4+. Once the nitrogen enters the estuaries via groundwater, it is thought that because there is more 15N entering, that there will also be more 15N in the inorganic nitrogen pool delivered and that it is picked up more by producers taking up N. Even though 14N is easier to take up, because there is much more 15N, there will still be higher amounts assimilated than normal. These levels of δ15N can be examined in creatures that live in the area and are non migratory (such as macrophyte
Macrophyte
A macrophyte is an aquatic plant that grows in or near water and is either emergent, submergent, or floating. In lakes macrophytes provide cover for fish and substrate for aquatic invertebrates, produce oxygen, and act as food for some fish and wildlife....
s, clams and even some fish). This method of identifying high levels of nitrogen input is becoming a more and more popular method in attempting to monitor nutrient input into estuaries and coastal ecosystems. Environmental managers have become more and more concerned about measuring anthropogenic nutrient inputs into estuaries because excess in nutrients can lead to eutrophication
Eutrophication
Eutrophication or more precisely hypertrophication, is the movement of a body of water′s trophic status in the direction of increasing plant biomass, by the addition of artificial or natural substances, such as nitrates and phosphates, through fertilizers or sewage, to an aquatic system...
and hypoxic events
Hypoxia (environmental)
Hypoxia, or oxygen depletion, is a phenomenon that occurs in aquatic environments as dissolved oxygen becomes reduced in concentration to a point where it becomes detrimental to aquatic organisms living in the system...
, eliminating organisms from an area entirely.
Forensics
A recent development in forensic science is the isotopic analysis of hair strands. Hair has a recognisable growth rate of 9-11mm per month or 15 cm per year. Hair growth is primarily a function of diet, especially drinking water intake. The stable isotopic ratios of drinking water are a function of location, and the geology that the water percolates through. 87Sr, 88Sr and Oxygen isotope variations are different all over the World. These differences in isotopic ratio are then biologically 'set' in our hair as it grows and it has therefore become possible to identify recent geographic histories by the analysis of hair strands. For example, it could be possible to identify whether a terrorist suspect had recently been to a particular location from hair analysis. This hair analysis is a non-invasive method which is becoming very popular in cases that DNA or other traditional means are bringing no answers.Isotope analysis can be used by forensic investigators to determine whether two or more samples of explosives are of a common origin. Most high explosives
Explosive material
An explosive material, also called an explosive, is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure...
contain carbon, hydrogen, nitrogen and oxygen atoms and thus comparing their relative abundances of isotopes can reveal the existence of a common origin. Researchers have also shown that analysis of the 12C/13C ratios can locate the country of origin for a given explosive.
Stable isotopic analysis has also been used in the identification of drug trafficking routes. Isotopic abundances are different in morphine grown from poppies in South-east Asia versus poppies grown in South-West Asia. The same is applied to cocaine that is derived from Bolivia and that from Columbia.
Paleoclimatology
The ratio of 18O to 16O in ice and deep sea cores is temperature dependent, and can be used as a proxy measure for reconstructing climate change. During colder periods of the Earth's history (glacials) such as during the ice ageIce age
An ice age or, more precisely, glacial age, is a generic geological period of long-term reduction in the temperature of the Earth's surface and atmosphere, resulting in the presence or expansion of continental ice sheets, polar ice sheets and alpine glaciers...
s, 16O is preferentially evaporated from the colder oceans, leaving the slightly heavier and more sluggish 18O behind. Organisms such as foraminifera which combine oxygen dissolved in the surrounding water with carbon and calcium to build their shells therefore incorporate the temperature-dependent 18O to 16O ratio. When these organisms die, they settle out on the sea bed, preserving a long and invaluable record of global climate change through much of the Quaternary
Quaternary
The Quaternary Period is the most recent of the three periods of the Cenozoic Era in the geologic time scale of the ICS. It follows the Neogene Period, spanning 2.588 ± 0.005 million years ago to the present...
. Similarly, ice cores on land are enriched in the heavier 18O relative to 16O during warmer climatic phases (interglacial
Interglacial
An Interglacial period is a geological interval of warmer global average temperature lasting thousands of years that separates consecutive glacial periods within an ice age...
s) as more energy is available for the evaporation of the heavier 18O isotope. The oxygen isotope record preserved in the ice cores is therefore a `mirror` of the record contained in ocean sediments.
Oxygen isotopes preserve a record of the effects of the Milankovitch cycles
Milankovitch cycles
Milankovitch theory describes the collective effects of changes in the Earth's movements upon its climate, named after Serbian civil engineer and mathematician Milutin Milanković, who worked on it during First World War internment...
on climate change during the Quaternary, revealing an approximately 100,000-year cyclicity in the Earth's climate.
External links
- IsoSource. Stable isotope mixing model for an excess number of sources (Visual Basic), (Phillips and Gregg, 2003).
- MixSIR: A Bayesian Stable Isotope Mixing Model. MixSIR is a free graphical user interface (GUI) program built on the MATLAB platform that carries out Bayesian analysis of stable isotope mixing models using sampling-importance-resampling (SIR). (Moore and Semmens, 2008).
- SIAR - Stable isotope analysis in R.. Bayesian mixing model package for the R environment. Parnell, A., Inger, R., Bearhop, S., Jackson, A.
- SISUS: Stable Isotope Sourcing using Sampling. Stable Isotope Sourcing using Sampling (SISUS) (Erhardt, Wolf, and Bedrick, In Prep.) provides a more efficient algorithm to provide solutions to the same problem as the Phillips and Gregg (2003) IsoSource model and software for source partitioning using stable isotopes.

