
Oxygen-18
Encyclopedia
Oxygen-18 is a natural, stable
isotope
of oxygen
and one of the environmental isotopes
.
18O is an important precursor for the production of fluorodeoxyglucose
(FDG) used in positron emission tomography
(PET). Generally, in the radiopharmaceutical industry, enriched water (H218O) is bombarded with hydrogen ions in either a cyclotron
or linear accelerator
creating fluorine-18
. This is then synthesized into FDG and injected into a patient. It can also be used to make an extremely heavy version of water, 3H218O, or T218O. This compound has a density almost 30% greater than natural water
and Antarctic
, the ratio O-18/O-16 (δ18O) can be used to determine the temperature of precipitation through time. Assuming that atmospheric circulation and elevation has not changed significantly over the poles, the temperature of ice formation can be calculated as equilibrium fractionation between phases of water that is known for different temperatures. Water molecules are also subject to Rayleigh fractionation as atmospheric water moves from the equator poleward which results in progressive depletion of O-18, or lower δ18O values. In the 1950s, Harold Urey
performed an experiment in which he mixed both normal water and water with oxygen-18 in a barrel, and then partially froze the barrel's contents.
The ratio O-18/O-16 (δ18O) can also be used to determine paleothermometry in certain types of fossils. The fossils in question have to show progressive growth in the animal or plant that the fossil represents. The fossil material used is generally calcite
or aragonite
, however oxygen isotope paleothermometry has also been done of phosphatic
fossils using SHRIMP . For example, seasonal temperature variations may be determined from a single sea shell from a scallop
. As the scallop grows, an extension is seen on the surface of the shell. Each growth band can be measured, and a calculation is used to determine the probable sea water temperature in comparison to each growth. The equation for this is:
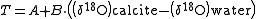
Where T is temperature in Celsius and A and B are constants.
For determination of ocean temperatures over geologic time, multiple fossils of the same species in different stratigraphic layers
would be measured, and the difference between them would indicate long term changes.
, the labeling of atmosphere by oxygen-18 allows us to measure the oxygen uptake by the photorespiration pathway. Labeling by 18O2 gives the unidirectional flux of O2 uptake, while there is a net photosynthetic 16O2 evolution. It was demonstrated that, under preindustrial atmosphere, most plants reabsorb, by photorespiration, half of the oxygen produced by photosynthesis
. Then, the yield of photosynthesis was halved by the presence of oxygen in atmosphere.
Stable isotope
Stable isotopes are chemical isotopes that may or may not be radioactive, but if radioactive, have half-lives too long to be measured.Only 90 nuclides from the first 40 elements are energetically stable to any kind of decay save proton decay, in theory...
isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
of oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and one of the environmental isotopes
Environmental isotopes
The environmental isotopes are a subset of the isotopes, both stable and radioactive, which are the object of Isotope geochemistry.The most used environmental isotopes are:* deuterium* tritium* carbon-13* carbon-14* nitrogen-15* oxygen-18...
.
18O is an important precursor for the production of fluorodeoxyglucose
Fluorodeoxyglucose
Fludeoxyglucose or fluorodeoxyglucose , commonly abbreviated 18F-FDG or FDG, is a radiopharmaceutical used in the medical imaging modality positron emission tomography...
(FDG) used in positron emission tomography
Positron emission tomography
Positron emission tomography is nuclear medicine imaging technique that produces a three-dimensional image or picture of functional processes in the body. The system detects pairs of gamma rays emitted indirectly by a positron-emitting radionuclide , which is introduced into the body on a...
(PET). Generally, in the radiopharmaceutical industry, enriched water (H218O) is bombarded with hydrogen ions in either a cyclotron
Cyclotron
In technology, a cyclotron is a type of particle accelerator. In physics, the cyclotron frequency or gyrofrequency is the frequency of a charged particle moving perpendicularly to the direction of a uniform magnetic field, i.e. a magnetic field of constant magnitude and direction...
or linear accelerator
Linear particle accelerator
A linear particle accelerator is a type of particle accelerator that greatly increases the velocity of charged subatomic particles or ions by subjecting the charged particles to a series of oscillating electric potentials along a linear beamline; this method of particle acceleration was invented...
creating fluorine-18
Fluorine-18
Fluorine-18 is a fluorine radioisotope which is an important source of positrons. It has a mass of 18.0009380 u and its half-life is 109.771 minutes....
. This is then synthesized into FDG and injected into a patient. It can also be used to make an extremely heavy version of water, 3H218O, or T218O. This compound has a density almost 30% greater than natural water
Paleoclimatology
In ice cores, mainly ArcticArctic
The Arctic is a region located at the northern-most part of the Earth. The Arctic consists of the Arctic Ocean and parts of Canada, Russia, Greenland, the United States, Norway, Sweden, Finland, and Iceland. The Arctic region consists of a vast, ice-covered ocean, surrounded by treeless permafrost...
and Antarctic
Antarctic
The Antarctic is the region around the Earth's South Pole, opposite the Arctic region around the North Pole. The Antarctic comprises the continent of Antarctica and the ice shelves, waters and island territories in the Southern Ocean situated south of the Antarctic Convergence...
, the ratio O-18/O-16 (δ18O) can be used to determine the temperature of precipitation through time. Assuming that atmospheric circulation and elevation has not changed significantly over the poles, the temperature of ice formation can be calculated as equilibrium fractionation between phases of water that is known for different temperatures. Water molecules are also subject to Rayleigh fractionation as atmospheric water moves from the equator poleward which results in progressive depletion of O-18, or lower δ18O values. In the 1950s, Harold Urey
Harold Urey
Harold Clayton Urey was an American physical chemist whose pioneering work on isotopes earned him the Nobel Prize in Chemistry in 1934...
performed an experiment in which he mixed both normal water and water with oxygen-18 in a barrel, and then partially froze the barrel's contents.
The ratio O-18/O-16 (δ18O) can also be used to determine paleothermometry in certain types of fossils. The fossils in question have to show progressive growth in the animal or plant that the fossil represents. The fossil material used is generally calcite
Calcite
Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate . The other polymorphs are the minerals aragonite and vaterite. Aragonite will change to calcite at 380-470°C, and vaterite is even less stable.-Properties:...
or aragonite
Aragonite
Aragonite is a carbonate mineral, one of the two common, naturally occurring, crystal forms of calcium carbonate, CaCO3...
, however oxygen isotope paleothermometry has also been done of phosphatic
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
fossils using SHRIMP . For example, seasonal temperature variations may be determined from a single sea shell from a scallop
Scallop
A scallop is a marine bivalve mollusk of the family Pectinidae. Scallops are a cosmopolitan family, found in all of the world's oceans. Many scallops are highly prized as a food source...
. As the scallop grows, an extension is seen on the surface of the shell. Each growth band can be measured, and a calculation is used to determine the probable sea water temperature in comparison to each growth. The equation for this is:
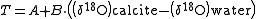
Where T is temperature in Celsius and A and B are constants.
For determination of ocean temperatures over geologic time, multiple fossils of the same species in different stratigraphic layers
Stratigraphy
Stratigraphy, a branch of geology, studies rock layers and layering . It is primarily used in the study of sedimentary and layered volcanic rocks....
would be measured, and the difference between them would indicate long term changes.
Plant physiology
In the study of plants photorespirationPhotorespiration
Photorespiration, or "'photo-respiration'", is a process in plant metabolism by which RuBP has oxygen added to it by the enzyme , instead of carbon dioxide during normal photosynthesis. This is the beginning step of the Calvin-Benson cycle...
, the labeling of atmosphere by oxygen-18 allows us to measure the oxygen uptake by the photorespiration pathway. Labeling by 18O2 gives the unidirectional flux of O2 uptake, while there is a net photosynthetic 16O2 evolution. It was demonstrated that, under preindustrial atmosphere, most plants reabsorb, by photorespiration, half of the oxygen produced by photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
. Then, the yield of photosynthesis was halved by the presence of oxygen in atmosphere.
See also
- Willi DansgaardWilli DansgaardWilli Dansgaard was a Danish paleoclimatologist. He was Professor Emeritus of Geophysics at the University of Copenhagen and a member of the Royal Danish Academy of Science and Letters, the Royal Swedish Academy of Sciences, the Icelandic Academy of Sciences, and the Danish Geophysical Society.-...
- a paleoclimatologistPaleoclimatologyPaleoclimatology is the study of changes in climate taken on the scale of the entire history of Earth. It uses a variety of proxy methods from the Earth and life sciences to obtain data previously preserved within rocks, sediments, ice sheets, tree rings, corals, shells and microfossils; it then... - Isotopes of oxygenIsotopes of oxygenThere are three stable isotopes of oxygen that lead to oxygen having a standard atomic mass of 15.9994 u. 17 radioactive isotopes have also been characterized, with mass numbers from 12O to 28O, all short-lived, with the longest-lived being 15O with a half-life of 122.24 seconds...
- Paleothermometry
- Δ18O

