IUPAC nomenclature of inorganic chemistry 2005
Encyclopedia
The Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005, commonly known as The Red Book, is a collection of rules for naming inorganic compounds, as recommended by the IUPAC.
of Inorganic Chemistry, IUPAC Recommendations 1990 (Red Book I)", and "where appropriate" (sic) "Nomenclature of Inorganic Chemistry II, IUPAC Recommendations 2000 (Red Book II)".
The recommendations take up over 300 pages and the full text can be downloaded from IUPAC. Corrections have been issued.
Apart from a reorganisation of the content, there is a new section on organometallics and a formal element list to be used in place of electronegativity
lists in sequencing elements in formulae and names. The concept of a preferred IUPAC name (PIN), a part of the revised blue book for organic compound naming, has not yet been adopted for inorganic compounds. There are however guidelines as to which naming method should be adopted.
Additionally there are recommendations for the following:
For a simple compound such as AlCl3
the different naming conventions yield the following:
A simple rule of thumb ignoring lanthanides and actinides is:
The full list, from highest to lowest "electronegativity":
Note "treat separately" means to use the decision table on each component
for the crystal form.(Note that the recommendations specifically italicise the second character.
Pn,. red phosphorus ; Asn, amorphous arsenic.
Stoichiometric names are the simplest and reflect either the empirical formula or the molecular formula. The ordering of the elements follows the formal electronegativity list for binary compounds and electronegativity list to group the elements into two classes which are then alphabetically sequenced. The proportions are specified by di-, tri-, etc. (See IUPAC numerical multiplier
.) Where there are known to be complex cations or anions these are named in their own right and then these names used as part of the compound name.
Taking the binary compound of sodium and chlorine: chlorine is found first in the list so therefore comes last in the name. Other examples are
The 1:1:1:1 quaternary compound between bromine chlorine iodine and phosphorus:
The ternary 2:1:5 compound of antimony, copper and potassium can be named in two ways depending on which element(s) are designated as electronegative.
Sometimes an abbreviated from of the element name has to be taken , e.g. germide for germanium as germanide refers to GeH3− .
Polyatomic cations of the same element are named as the element name preceded by di-, tri-, etc.
, e.g.:
Polyatomic cations made up of different elements are named either substitutively or additively, e.g.:
− ) e.g.:
Some elements take their Latin name as the root e.g
Polyatomic anions of the same element are named as the element name preceded by di-, tri-, etc.
, e.g.:
or sometimes as an alternative derived from a substitutive name e.g.
Polyatomic anions made up of different elements are named either substitutively or additively, the name endings are -ide and -ate respectively e.g. :
A full list of the alternative acceptable non-systematic names for cations and anions is in the recommendations.
Many anions have names derived from inorganic acids and these are dealt with later.
· ". For example:
· 10H2O, sodium sulfate decahydrate. The recommended method would be to name it sodium sulfate—water(1/10). Similarly other examples of lattice compounds are:
Substitutive nomenclature =
This naming method generally follows established IUPAC organic nomenclature. Hydrides of the main group elements (groups 13-17) are given -ane base names, e.g. borane, BH3. Acceptable alternative names for some of the parent hydrides are water rather than oxidane and ammonia rather than azane. In these cases the base name is intended to be used for substituted derivatives.
This section of the recommendations covers the naming of compounds containing rings and chains.
is added to the parent hydride name. Examples are:
There is a fully systematic method of numbering the atoms in the boron hydride clusters, and a method of describing the position of bridging hydrogen atoms using the μ symbol.
For organometallic compounds of groups 1-2 can use additive (indicating a molecular aggregate) or compositional naming. Examples are:
However the recommendation notes that future nomenclature projects will be addressing these compounds.
Additive nomenclature=
This naming has been developed principally for coordination compounds although it can be more widely applied. Examples are:
− becomes chlorido. This is a difference from organic compound naming and substitutive naming where chlorine is treated as neutral and it becomes chloro, as in PCl3, which can be named as either substitutively or additively as trichlorophosphane or trichloridophosphorus respectively.
Similarly if the anion names end in -ite, -ate then the ligand names are -ito, -ato.
for simple ligands or bis-, tris-, tetrakis-,etc for complex ligands. For example:
The central atom name(s) come after the ligands. Where there is more than one central atom it is preceded by di- tri-, tetra- etc.
Where there are different central atoms they are sequenced using the electronegativity list.
This example illustrates the ordering of bridging and non bridging ligands of the same type. In the formula the bridging ligands follow the non bridging whereas in the name the bridging ligands precede the non bridging. Note the use of the kappa convention to specify that there are two terminal chlorides on each aluminium.
which can be visualised as a tetrahedral arrangement of Be atoms linked by 6 acetate ions forming a cage with a central oxide anion, the formula and name are as follows:
The μ4 describing the bridging of the central oxide ion. (Note the use of the kappa convention to describe the bridging of the acetate ion where both oxygen atoms are involved.)
In the name where a ligand is involved in different modes of bridging, the multiple bridging is listed in decreasing order of complexity,
e.g. μ3 bridging before μ2 bridging.
For monodentate ligands there is no ambiguity as to which atom is forming the bond to the central atom. However when a ligand has more than one atom that can link to a central atom the kappa convention is used to specify which atoms in a ligand are forming a bond. The element atomic symbol is italicised and preceded by kappa,κ. These symbols are placed after the portion of the ligand name that represents the ring, chain etc where the ligand is located. For example:
Where there is more than one bond formed from a ligand by a particular elemnt aq numerical superscript gives the count. For example:
In polynuclear complexes the use of the kappa symbol is extended in two related ways. Firstly to specify which ligating atoms bind to which central atom and secondly to specify for a bridging ligand which central atoms are involved. The central atoms must be identified, i.e. by assigning numbers to them. (This is formally dealt with in the recommendations).
To specify which ligating atoms in a ligand link to which central atom, the central atom numbers precede the kappa symbol, and numerical superscript specifies the number of ligations and this is followed by the atomic symbol. Multiple occurrences are separated by commas.
Examples:
is used to describe the geometry. A configuration index
is determined from the positions of the ligands and together with the polyhedral symbol
is placed at the beginning of the name. For example in the complex (SP-4-3)-(acetonitrile)dichlorido(pyridine)platinum(II) the (SP-4-3) at the beginning of the name describes a square planar geometry, 4 coordinate with a configuration index of 3 indicating the position of the ligands around the central atom. For more detail see polyhedral symbol
.
Examples of compounds that meet the criteria are:
Examples of compounds that should not be named as metallocenes are:
(3 Os—Os) in Decacarbonyldihydridotriosmium
A pair of brackets contain a count of the bonds formed (if greater than 1), followed by the italicised element atomic symbols separated by an "em-dash".
Examples:
decacarbonyldimanganese10.png)
bis(pentacarbonylmanganese)(Mn—Mn)
dodecacarbonyltetrarhodium12.png)
tri-μ-carbonyl-1:2κ2C;1:3κ2C;2:3κ2C-nonacarbonyl-
1κ2C,2κ2C,3κ2C,4κ3C-[Td-(13)-Δ4-closo]-tetrarhodium(6 Rh—Rh)
or tri-μ-carbonyl-1:2κ2C;1:3κ2C;2:3κ2C-nonacarbonyl-
1κ2C,2κ2C,3κ2C,4κ3C-tetrahedro-tetrarhodium(6 Rh—Rh)
Note that the difference from the compositional naming method (hydrogen sulfide) as in hydrogen naming there is NO space between the electropositive and electronegative components.
This method gives no structural information regarding the position of the hydrons (hydrogen atoms). If this information is to be conveyed then the additive name should be used (see the list below for examples).
Where there is a continuous range of composition this can be written e.g., K(Br,Cl) for a mixture of KBr and KCl and (Li2,Mg)Cl2 for a mixture of LiCl and MgCl2.
The recommendation is to use the following generalised method e.g.
Note that cation vacancies in CoO could be described by CoO1-x
, note that the IUPAC preference is for vacancies to be specified by V rather than V (the element vanadium).
may be used. The use of "Strukturbericht" (e.g. A1 etc) or Greek letters is not acceptable. The Pearson symbol may be followed by the space group and the prototype formula. Examples are:
Summary
The 2005 edition replaces their previous recommendations "Nomenclature The Red Bookof Inorganic Chemistry, IUPAC Recommendations 1990 (Red Book I)", and "where appropriate" (sic) "Nomenclature of Inorganic Chemistry II, IUPAC Recommendations 2000 (Red Book II)".
The recommendations take up over 300 pages and the full text can be downloaded from IUPAC. Corrections have been issued.
Apart from a reorganisation of the content, there is a new section on organometallics and a formal element list to be used in place of electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
lists in sequencing elements in formulae and names. The concept of a preferred IUPAC name (PIN), a part of the revised blue book for organic compound naming, has not yet been adopted for inorganic compounds. There are however guidelines as to which naming method should be adopted.
Naming methods
The recommendations describe a number of different ways in which compounds can be named. These are:- compositional naming (e.g. sodium chloride)
- substitutive naming based on parent hydrides (GeCl2Me2 dichlorodimethylgermane)
- additive naming ([MnFO3] fluoridotrioxidomanganese)
Additionally there are recommendations for the following:
- naming of cluster compounds
- allowed names for inorganic acids and derivatives
- naming of solid phases e.g. non-stoichiometric phases
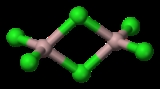
For a simple compound such as AlCl3
Aluminium chloride
Aluminium chloride is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large...
the different naming conventions yield the following:
- compositional: aluminium trichloride (stoichiometricallyStoichiometryStoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
) or dialuminium hexachloride (dimer) - substitutional: trichloralumane
- additive: trichloridoaluminium; hexachloridoaluminium (dimer without structural information); di-μ-chlorido-tetrachlorido-1κ2Cl,2κ2Cl-dialuminium (dimer with structural information)
Sequencing elements - the "electronegativity" list
Throughout the recommendations the use of the electronegativity of elements for sequencing has been replaced by a formal list which is loosely based on electronegativity. The recommendations still use the terms electropositive and electronegative to refer to an element's relative position in this list.A simple rule of thumb ignoring lanthanides and actinides is:
- for two elements in different groups - then the element in the higher numbered group has higher "electronegativity"
- for two elements within the same group the element with the lower the atomic number has the higher "electronegativity"
- Hydrogen is fitted in to be less electronegative than polonium and more electronegative than nitrogen. Hence the formulae of water and ammonia can be written H2O and NH3 respectively.
The full list, from highest to lowest "electronegativity":
- Group 17 in atomic number sequence i.e. F-At followed by
- Group 16 in atomic number sequence i.e. O-Po followed by
- H, hydrogenHydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, followed by - Group 15 in atomic number sequence i.e. N-Bi followed by
- Group 14 in atomic number sequence i.e. C-Pb followed by
- Group 13Boron groupThe boron group is the series of elements in group 13 of the periodic table, comprising boron , aluminium , gallium , indium , thallium , and ununtrium . The elements in the boron group are characterized by having three electrons in their outer energy levels...
in atomic number sequence i.e. B-Tl followed by - Group 12Group 12 elementA group 12 element is one of the elements in group 12 in the periodic table. This includes zinc , cadmium and mercury . The further inclusion of copernicium in group 12 is supported by recent experiments on individual Cn atoms...
in atomic number sequence i.e. Zn-Cn followed by - Group 11 in atomic number sequence i.e. Cu-Rg followed by
- Group 10 in atomic number sequence i.e. Ni-Ds followed by
- Group 9Group 9 elementIn modern IUPAC nomenclature, Group 9 of the periodic table contains the elements cobalt , rhodium , iridium , and meitnerium . These are all d-block transition metals...
in atomic number sequence i.e. Co-Mt followed by - Group 8Group 8 elementA Group 8 element is one in the series of elements in group 8 in the periodic table, which consists of the transition metals iron , ruthenium , osmium and hassium ....
in atomic number sequence i.e. Fe-Hs followed by - Group 7Group 7 elementA Group 7 element is one in the series of elements in group 7 in the periodic table, which consists of manganese , technetium , rhenium , and bohrium...
in atomic number sequence i.e. Mn-Bh followed by - Group 6Group 6 elementA Group 6 element is one in the series of elements in group 6 in the periodic table, which consists of the transition metals chromium , molybdenum , tungsten , and seaborgium ....
in atomic number sequence i.e. Cr-Sg followed by - Group 5Group 5 elementA Group 5 element is a chemical element in the fifth group in the periodic table. In the modern IUPAC nomenclature, Group 5 of the periodic table contains vanadium , niobium , tantalum and dubnium . This group lies in the d-block of the periodic table...
in atomic number sequence i.e. V-Db followed by - Group 4Group 4 elementThe Group 4 elements are a group of chemical elements in the periodic table. In the modern IUPAC nomenclature, Group 4 of the periodic table contains titanium , zirconium , hafnium and rutherfordium . This group lies in the d-block of the periodic table...
in atomic number sequence i.e. Ti-Rf followed by - Group 3Group 3 elementThe group 3 elements are a group of chemical elements in the periodic table. This group, like other d-block groups, should contain four elements, but it is not agreed what elements belong in the group...
in atomic number sequence i.e. Sc-La followed by - the lanthanoids in atomic number sequence i.e. La-Lu followed by
- the actinoids in atomic number sequence i.e. Ac-Lr followed by
- Group 2Alkaline earth metalThe alkaline earth metals are a group in the periodic table. In the modern IUPAC nomenclature, the alkaline earth metals are called the group 2 elements. Previously, they were called the Group IIA elements . The alkaline earth metals contain beryllium , magnesium , calcium , strontium , barium and...
in atomic number sequence i.e. Be-Ra followed by - Group 1 (excluding H)Alkali metalThe alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
in atomic number sequence i.e. Li-Fr followed by - Group 18Noble gasThe noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
in atomic number sequence i.e. He-Rn
Determining the nomenclature to use
| Action | Addition compound? | Definite stoichiometry? | mono-atomic? | molecular Molecule A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge... ? | metal Metal A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light... present? | Bond to carbon Carbon Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds... ? | transition metal Transition metal The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some... group3-12? | main group metal groups 1,2,3-6? |
|---|---|---|---|---|---|---|---|---|
| Treat each component separately use compositional |
Y | |||||||
| Use solids naming | N | N | ||||||
| Element or monoatomic cation/anion/radical naming | N | Y | Y | |||||
| Divide components into "electropositive"/"electronegative" Treat each component separately Use generalised stoichiometric naming |
N | Y | N | N | ||||
| Use Blue book (Organic compound Organic compound An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the... ) |
' N | Y | N | Y | N | Y | ||
| Use additive naming for group 3- 12 organometallics Organometallic chemistry Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character... |
N | Y | N | Y | Y | Y | Y | |
| Use substitutive naming for group 3- 6 organometallics Use compositional for groups 1-2 organometallics |
N | Y | N | Y | Y | Y | N | Y |
| Use additive naming for coordination complexes | N | Y | N | Y | Y | N | Y | |
| Choose either substitutive or additive | N | Y | N | Y | N | N |
Note "treat separately" means to use the decision table on each component
Sample of indeterminate structure
An indeterminate sample simply takes the element name. For example a sample of carbon (which could be diamond, graphite etc or a mixture) would be named carbon.Molecular
- O2 dioxygen (acceptable name oxygen)
- O3 trioxygen (acceptable name ozone)
- P4 tetraphosphorus (acceptable name white phosphorus)
- S6 hexasulfur (acceptable name ε-sulfur)
- S8 cyclo-octasulfur (acceptable names for the polymorphic forms are α-sulfur, β-sulfur, γ-sulfur)
Crystalline form
This is specified by the element symbol followed by the Pearson symbolPearson symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W.B. Pearson. The symbol is made up of two letters followed by a number. For example:* Diamond structure, cF8...
for the crystal form.(Note that the recommendations specifically italicise the second character.
- Cn carbon(cF8) (acceptable name diamond)
- Snn tin(tI4) (acceptable name β- or white tin)
- Mnn manganese(cI58) (acceptable name α-manganese)
Amorphous recognised allotropes
Examples includePn,. red phosphorus ; Asn, amorphous arsenic.
Compounds
Compositional names impart little structural information and are recommended for use when structural information is not available or does not need to be conveyed.Stoichiometric names are the simplest and reflect either the empirical formula or the molecular formula. The ordering of the elements follows the formal electronegativity list for binary compounds and electronegativity list to group the elements into two classes which are then alphabetically sequenced. The proportions are specified by di-, tri-, etc. (See IUPAC numerical multiplier
IUPAC numerical multiplier
The numerical multiplier in IUPAC nomenclature indicates how many particular atoms or functional groups are attached at a particular point in a molecule...
.) Where there are known to be complex cations or anions these are named in their own right and then these names used as part of the compound name.
Binary compounds
In binary compounds the more electropositive element is placed first in the formula. The formal list is used. The name of the most electronegative element is modified to end in -ide and the more electropositive elements name is left unchanged.Taking the binary compound of sodium and chlorine: chlorine is found first in the list so therefore comes last in the name. Other examples are
- PCl5 phosphorus pentachloride
- Ca2P3 dicalcium triphosphide
- NiSn nickel stannide
- Cr23C6 tricosachromium hexacarbide
Ternary compounds and beyond
The following illustrate the principles.The 1:1:1:1 quaternary compound between bromine chlorine iodine and phosphorus:
- PBrClI phosphorus bromide chloride iodide (phosphorus is the most electropositive, the others are all designated as electronegative and are sequenced alphabetically)
The ternary 2:1:5 compound of antimony, copper and potassium can be named in two ways depending on which element(s) are designated as electronegative.
- CuK5Sb2 copper pentapotassium diantimonide, (both copper and potassium are designated as electropositive and are sequenced alphabetically)
- K5CuSb2 pentapotassium diantimonide cupride (only potassium is designated as electropositive and the two electronegative elements are sequenced alphabetically) (Note the red book shows this example incorrectly)
Cations
Monoatomic cations are named by taking the element name and following it with the charge in brackets e.g- Na+ sodium(1+)
- Cr3+ chromium(3+)
Sometimes an abbreviated from of the element name has to be taken , e.g. germide for germanium as germanide refers to GeH3
Polyatomic cations of the same element are named as the element name preceded by di-, tri-, etc.
IUPAC numerical multiplier
The numerical multiplier in IUPAC nomenclature indicates how many particular atoms or functional groups are attached at a particular point in a molecule...
, e.g.:
- Hg22+ dimercury(2+)
Polyatomic cations made up of different elements are named either substitutively or additively, e.g.:
- PH4+ phosphanium
- SbF4+ tetrafluorostibanium (substitutive) or tetrafluoridoantimony(1+)
- Note that ammonium and oxonium are acceptable names for NH4+ and H3O+ respectively. (Hydronium is not an acceptable name for H3O+ )
Anions
Monatomic anions are named as the element modified with an -ide ending. The charge follows in brackets, (optional for 1- Cl
− chloride(1− ) or chloride - S2
− sulfide(2− )
Some elements take their Latin name as the root e.g
- silver, Ag, argentide
- copper, Cu, cupride
- iron, Fe, ferride
- tin, Sn, stannide
Polyatomic anions of the same element are named as the element name preceded by di-, tri-, etc.
IUPAC numerical multiplier
The numerical multiplier in IUPAC nomenclature indicates how many particular atoms or functional groups are attached at a particular point in a molecule...
, e.g.:
- O22
− dioxide(2− ) (or peroxide as an acceptable name) - C22
− dicarbide(2− ) (or acetylide as an acceptable name) - S22
− disulfide(2− )
or sometimes as an alternative derived from a substitutive name e.g.
- S22
− disulfanediide
Polyatomic anions made up of different elements are named either substitutively or additively, the name endings are -ide and -ate respectively e.g. :
- GeH3
− germanide (substitutive), or trihydridogermanate(1− ) (additive) - TeH3
− tellanuide substitutive where -uide specifies anion composed of additional hydride attached to parent hydride - [PF6]
− hexafluoro-λ5-phosphanuide (substitutive), or hexafluoridophosphate(1− ) (additive) - SO32
− trioxidosulfate(2− ) (additive), or sulfite (acceptable non-systematic name)
A full list of the alternative acceptable non-systematic names for cations and anions is in the recommendations.
Many anions have names derived from inorganic acids and these are dealt with later.
Radicals
The presence of unpaired electrons can be indicated by a "- He
· + helium(· +) - N2(2
· )2+ dinitrogen(2· 2+)
Naming of hydrates and similar lattice compounds
The use of the term hydrate is still acceptable e.g. Na2SO4- CaCl2
· 8NH3, calcium chloride— ammonia (1/8) - 2Na2CO3
· 3H2O2Sodium percarbonateSodium percarbonate is a chemical, an adduct of sodium carbonate and hydrogen peroxide , with formula Na2CO3 · 1.5H2O2. It is a colorless, crystalline, hygroscopic and water-soluble solid...
, sodium carbonate—hydrogen peroxide (2/3) - AlCl3
· 4EtOH, aluminium chloride—ethanol (1/4)
Specifying proportions using charge or oxidation state
As an alternative to di-, tri- prefixes either charge or oxidation state can be used. Charge is recommended as oxidation state may be ambiguous and open to debate.Substitutive nomenclature =
This naming method generally follows established IUPAC organic nomenclature. Hydrides of the main group elements (groups 13-17) are given -ane base names, e.g. borane, BH3. Acceptable alternative names for some of the parent hydrides are water rather than oxidane and ammonia rather than azane. In these cases the base name is intended to be used for substituted derivatives.
This section of the recommendations covers the naming of compounds containing rings and chains.
Base hydrides
| BH3 | borane | CH4 | methane | NH3 | azane (ammonia) |
H2O | oxidane (water) |
HF | fluorane (hydrogen fluoride) |
| AlH3 | alumane | SiH4 | silane | PH3 | phosphane | H2S | sulfane (hydrogen sulfide or dihydrogen sulfide) |
HCl | chlorane (hydrochloric acid) |
| GaH3 | gallane | GeH4 | germane | AsH3 | arsane | H2Se | selane | HBr | bromane (hydrobromic acid) |
| InH3 | indigane | SnH4 | stannane | SbH3 | stibane | H2Te | tellane (hydrogen telluride or dihydrogen telluride) |
HI | iodane (hydroiodic acid) |
| TlH3 | thallane | PbH4 | plumbane | BiH3 | bismuthane | H2Po | polane (hydrogen polonide or dihydrogen polonide) |
HAt | astatane |
Hydrides with non-standard bonding - lambda convention
Where a compound has non standard bonding as compared to the parent hydride for example PCl5 the lambda convention is used. For example:- PCl5 pentachloro-λ5-phosphane
- SF6 hexafluoro-λ6-sulfane
Polynuclear hydrides
A prefix di-, tri- etc.IUPAC numerical multiplier
The numerical multiplier in IUPAC nomenclature indicates how many particular atoms or functional groups are attached at a particular point in a molecule...
is added to the parent hydride name. Examples are:
- HOOH , dioxidane (hydrogen peroxide is an acceptable name)
- H2PPH2, diphosphane
- H3SiSiH2SiH2SiH3, tetrasilane
Rings and Chains
The recommendations describe three ways of assigning "parent" names to homonuclear monocyclic hydrides (i.e single rings consisting of one element):- the Hantzsch–Widman nomenclatureHantzsch–Widman nomenclatureHantzsch–Widman nomenclature, also called the extended Hantzsch–Widman system, is a type of systematic chemical nomenclature used for naming heterocyclic parent hydrides having no more than ten ring members....
(the method preferred for rings of size 3-10) - "skeletal replacement nomenclature" - specifying the replacement of carbon atoms in the corresponding carbon compound with atoms of another element (e.g. silicon becomes sila, germanium, germa) and a multiplicative prefix tri, tetra, penta etc)( the method preferred for rings greater than 10)
- by adding the prefix ‘cyclo’ to the name of the corresponding unbranched, unsubstituted chain
Boron hydrides
The stoichiometric name is followed by the number of hydrogen atoms in brackets. For example B2H6, diborane(6). More structural information can be conveyed by adding the "structural descriptor" closo-, nido-, arachno-, hypho-, klado- prefixes.There is a fully systematic method of numbering the atoms in the boron hydride clusters, and a method of describing the position of bridging hydrogen atoms using the μ symbol.
Main group organometallic compounds
Use of substitutive nomenclature is recommended for group 13-16 main group organometallic compounds. Examples are:- AlH2Me named methylalumane
- BiI2Ph named diiodo(phenyl)bismuthane
For organometallic compounds of groups 1-2 can use additive (indicating a molecular aggregate) or compositional naming. Examples are:
- [BeEtH] named ethylhydridoberyllium or ethanidohydridoberyllium
- [Mg(η5-C5H5)2] named bis(η5-cyclopentadienyl)magnesium, or bis(η5-cyclopentadienido)magnesium
- Na(CHCH2) sodium ethenide (compositional name)
However the recommendation notes that future nomenclature projects will be addressing these compounds.
Additive nomenclature=
This naming has been developed principally for coordination compounds although it can be more widely applied. Examples are:
- Si(OH)4 tetrahydroxidosilicon (additive),or silanetetrol (substitutive) (note silicic acid is an acceptable name - orthosilicic has been dropped).
- [CoCl(NH3)5]Cl2 pentaamminechloridocobalt(2+) chloride
Recommended procedure for naming mononuclear compounds
The recommendations include a flow chart which can be summarised very briefly:- identify the central atom,
- identify and name the ligands,
- specify coordination mode of ligands i.e. using kappa and/or eta conventions
- sequence the ligands
- specify coordination geometry i.e polyhedral symbol,configuration index (using CIP rules and absolute configuration for optically active compounds.
Anionic ligands
If the anion name ends in -ide then as a ligand its name is changed to end in -o. For example the chloride anion, ClSimilarly if the anion names end in -ite, -ate then the ligand names are -ito, -ato.
Neutral ligands
Neutral ligands do not change name with the exception of the following:- Water, "aqua"
- Ammonia, "ammine"
- Carbon monoxide bonded via carbon, "carbonyl"
- Nitrogen monoxide bonded via nitrogen, "nitrosyl"
Examples of ligand names
| Formula | name |
|---|---|
| Cl |
chlorido |
| CN |
cyanido |
| H |
hydrido |
| D |
deuterido or [2H]hydrido |
| PhCH2CH2Se |
2-phenylethane-1-selenolato |
| MeCOO |
acetato or ethanoato |
| Me2As |
dimethylarsanido |
| MePH |
methylphosphanido |
| MeCONH2 | acetamide (not acetamido) |
| MeCONH |
acetylazanido or acetylamido (not acetamido) |
| MeNH2 | methanamine |
| MeNH |
methylazanido, or methylamido, or methanaminido |
| MePH2 | methylphosphane |
| CO | carbonyl |
Sequence and position of ligands and central atoms
Ligands are ordered alphabetically by name and precede the central atom name. The number of ligands coordinating is indicated by the prefixes di-, tri-, tetra- penta- etc.IUPAC numerical multiplier
The numerical multiplier in IUPAC nomenclature indicates how many particular atoms or functional groups are attached at a particular point in a molecule...
for simple ligands or bis-, tris-, tetrakis-,etc for complex ligands. For example:
- [CoCl(NH3)5]Cl2 pentaamminechloridocobalt(2+) chloride where ammine (NH3)precedes chloride.
The central atom name(s) come after the ligands. Where there is more than one central atom it is preceded by di- tri-, tetra- etc.
- Os3(CO)12, dodecacarbonyltriosmium
Where there are different central atoms they are sequenced using the electronegativity list.
- [ReCo(CO)9] nonacarbonylrheniumcobalt
Bridging ligands- use of μ symbol
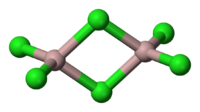
Ligands may bridge two or more centres. The prefix μ is used to specify a bridging ligand in both the formula and the name. For example the dimeric form of aluminium trichloride:
- Al2Cl4(μ-Cl)2
- di-μ-chlorido-tetrachlorido-1κ2Cl,2κ2Cl-dialuminium
This example illustrates the ordering of bridging and non bridging ligands of the same type. In the formula the bridging ligands follow the non bridging whereas in the name the bridging ligands precede the non bridging. Note the use of the kappa convention to specify that there are two terminal chlorides on each aluminium.
Bridging index
Where there are more than two centres that are bridged a bridging index is added as a subscript. For example in basic beryllium acetateBasic beryllium acetate
Basic beryllium acetate is the chemical compound with the formula Be4O6. Although this compound has no applications and has been only lightly studied, it adopts a distinctive structure. "Basic acetates" consist of an ensemble of metal atoms, a central oxide atom, and an exterior of acetate groups...
which can be visualised as a tetrahedral arrangement of Be atoms linked by 6 acetate ions forming a cage with a central oxide anion, the formula and name are as follows:
- [Be4(μ4-O)(μ-O2CMe)6]
- hexakis(μ-acetato-κO:kO′)-μ4-oxido-tetrahedro-tetraberyllium
The μ4 describing the bridging of the central oxide ion. (Note the use of the kappa convention to describe the bridging of the acetate ion where both oxygen atoms are involved.)
In the name where a ligand is involved in different modes of bridging, the multiple bridging is listed in decreasing order of complexity,
e.g. μ3 bridging before μ2 bridging.
Kappa,κ, convention
The kappa convention is used to specify which ligand atoms are bonding to the central atom and in polynuclear species which atoms , both bridged and unbridged link to which central atom.For monodentate ligands there is no ambiguity as to which atom is forming the bond to the central atom. However when a ligand has more than one atom that can link to a central atom the kappa convention is used to specify which atoms in a ligand are forming a bond. The element atomic symbol is italicised and preceded by kappa,κ. These symbols are placed after the portion of the ligand name that represents the ring, chain etc where the ligand is located. For example:
- pentaamminenitrito-κO-cobalt(III) specifies that the nitrite ligand is linking via the oxygen atom
Where there is more than one bond formed from a ligand by a particular elemnt aq numerical superscript gives the count. For example:
- aqua[(ethane-1,2-diyldinitrilo-κ2N,N’)tris(acetato-κO)acetato]cobaltate(1-), the cobalt anion formed with water and pentadentate edtaEDTAEthylenediaminetetraacetic acid, widely abbreviated as EDTA , is a polyamino carboxylic acid and a colourless, water-soluble solid. Its conjugate base is named ethylenediaminetetraacetate. It is widely used to dissolve limescale. Its usefulness arises because of its role as a hexadentate ligand...
, which links via two nitrogen atoms and three oxygen atoms. There are two bonds from nitrogen atoms in edta which is specified by -κ2N,N’. The three bonds from oxygen are specified by tris(acetato-κO), where there is one ligation per acetate.
In polynuclear complexes the use of the kappa symbol is extended in two related ways. Firstly to specify which ligating atoms bind to which central atom and secondly to specify for a bridging ligand which central atoms are involved. The central atoms must be identified, i.e. by assigning numbers to them. (This is formally dealt with in the recommendations).
To specify which ligating atoms in a ligand link to which central atom, the central atom numbers precede the kappa symbol, and numerical superscript specifies the number of ligations and this is followed by the atomic symbol. Multiple occurrences are separated by commas.
Examples:
- di-μ-chlorido-tetrachlorido-1κ2Cl,2κ2Cl-dialuminium , (aluminium trichloride).
- tetrachlorido-1κ2Cl,2κ2Cl specifies that there are two chloride ligands on each aluminium atom.
- decacarbonyl-1κ3C,2κ3C,3κ4C-di-μ-hydrido-1:2κ2H;1:2κ2H-triangulo-(3 Os—Os), (DecacarbonyldihydridotriosmiumDecacarbonyldihydridotriosmiumDecacarbonyldihydridotriosmium is a chemical compound with the formula H2Os310. This purple-violet crystalline air-stable cluster is noteworthy because it is electronically unsaturated and hence adds a variety of substrates.-Structure and synthesis:...
).- decacarbonyl-1κ3C,2κ3C,3κ4C shows that there are three carbonyl groups on two osmium atoms and four on the third.
- di-μ-hydrido-1:2κ2H;1:2κ2H specifies that the two hydride bridge between the osmium atom 1 and osmium atom 2.
Eta, η, convention
The use of η to denote hapticity is systematised. The use of η1 is not recommended. When the specification of the atoms involved is ambiguous the position of the atoms must be specified. This is illustrated by the examples:- Cr(η6-C6H6)2, named as bis(η6-benzene)chromium as all of the (contiguous) atoms in the benzene ligands are involved their position does not have to be specified
- [(1,2,5,6-η)-cycloocta-1,3,5,7-tetraene](η5-cyclopentadienyl)cobalt in this only two (at positions 1 and 5) of the four double bonds are linked to the central atom.
Coordination geometry
For any coordination number above 2 more than one coordination geometry is possible. For example four coordinate coordination compounds can be tetrahedral, square planar, square pyramidal or see-saw shaped. The polyhedral symbolPolyhedral symbol
The polyhedral symbol is sometimes used in coordination chemistry to indicate the approximate geometry of the coordinating atoms around the central atom. One or more italicised letters indicate the geometry, e.g. TP-3 which is followed by a number that gives the coordination number of the central...
is used to describe the geometry. A configuration index
Polyhedral symbol
The polyhedral symbol is sometimes used in coordination chemistry to indicate the approximate geometry of the coordinating atoms around the central atom. One or more italicised letters indicate the geometry, e.g. TP-3 which is followed by a number that gives the coordination number of the central...
is determined from the positions of the ligands and together with the polyhedral symbol
Polyhedral symbol
The polyhedral symbol is sometimes used in coordination chemistry to indicate the approximate geometry of the coordinating atoms around the central atom. One or more italicised letters indicate the geometry, e.g. TP-3 which is followed by a number that gives the coordination number of the central...
is placed at the beginning of the name. For example in the complex (SP-4-3)-(acetonitrile)dichlorido(pyridine)platinum(II) the (SP-4-3) at the beginning of the name describes a square planar geometry, 4 coordinate with a configuration index of 3 indicating the position of the ligands around the central atom. For more detail see polyhedral symbol
Polyhedral symbol
The polyhedral symbol is sometimes used in coordination chemistry to indicate the approximate geometry of the coordinating atoms around the central atom. One or more italicised letters indicate the geometry, e.g. TP-3 which is followed by a number that gives the coordination number of the central...
.
Organometallic groups 3-12
Additive nomenclature is generally recommended for organometallic compounds of groups 3-12 (transition metals and zinc, cadmium and mercury).Metallocenes
Following on from ferrocene - the first sandwich compound with a central Fe atom coordinated to two parallel cyclopentadienyl rings - names for compounds with similar structures such as osmocene and vanadocene are in common usage. The recommendation is that the name-ending ‘ocene’ should be restricted to compounds where there are discrete molecules of bis(η5-cyclopentadienyl)metal (and ring-substituted analogues), where the cyclopentadienyl rings are essentially parallel, and the metal is in the d-block. The terminology does NOT apply to compounds of the s- or p-block elements such as Ba(C5H5)2 or Sn(C5H5)2.Examples of compounds that meet the criteria are:
- vanadoceneVanadoceneVanadocene, bisvanadium, is the organometallic compound with the formula V2, commonly abbreviated Cp2V. It is a violet crystalline, paramagnetic solid...
, [V(η5-C5H5)2] - chromoceneChromoceneChromocene is an organochromium compound with the formula Cr2, often abbreviated Cp2Cr. This sandwich compound is structurally similar to ferrocene but does not follow the 18-electron rule because it only has 16 electrons. It also paramagnetic and highly reducing...
, [Cr(η5-C5H5)2] - cobaltoceneCobaltoceneCobaltocene, known also as biscobalt or even "bis Cp cobalt", is an organocobalt compound with the formula Co2. It is a dark purple solid that sublimes readily slightly above room temperature. Cobaltocene was discovered shortly after ferrocene, the first metallocene...
, [Co(η5-C5H5)2] - rhodoceneRhodoceneRhodocene, formally known as bisrhodium, is a chemical compound with the formula [Rh2]. Each molecule contains an atom of rhodium bound between two planar systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has covalent...
, [Rh(η5-C5H5)2] - nickeloceneNickeloceneNickelocene is the organonickel compound with the formula Ni2. Also known as bisnickel or NiCp2, this bright green paramagnetic solid is of enduring academic interest, although it yet has no practical applications....
, [Ni((η5-C5H5)2] - ruthenoceneRuthenoceneRuthenocene is an organoruthenium compound with the formula 2Ru. This pale yellow, volatile solid is classified as a sandwich compound and more specifically, as a metallocene.-Structure and bonding:...
, [Ru(η5-C5H5)2] - osmoceneOsmoceneOsmocene is an organoosmium compound found as a white solid. It is a metallocene.Osmocene is commercially available. It may be prepared by the reaction of osmium tetroxide with hydrobromic acid, followed by zinc and cyclopentadiene....
, [Os(η5-C5H5)2] - decamethylmanganocene, [Mn(η5-C5H5)2]
- [Re(η5-C5H5)2].
Examples of compounds that should not be named as metallocenes are:
- C10H10Ti
- [Ti(η5-C5H5)2Cl2] is properly named dichloridobis(η5-cyclopentadienyl)titanium NOT titanocene dichlorideTitanocene dichlorideTitanocene dichloride is the organotitanium compound with the formula 2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air...
Metal-metal bonds
In polynuclear compounds with metal- metal bonds these are shown after the element name as follows:(3 Os—Os) in Decacarbonyldihydridotriosmium
Decacarbonyldihydridotriosmium
Decacarbonyldihydridotriosmium is a chemical compound with the formula H2Os310. This purple-violet crystalline air-stable cluster is noteworthy because it is electronically unsaturated and hence adds a variety of substrates.-Structure and synthesis:...
A pair of brackets contain a count of the bonds formed (if greater than 1), followed by the italicised element atomic symbols separated by an "em-dash".
Polynuclear cluster geometry
The geometries of polynuclear clusters can range in complexity. A descriptor e.g. tetrahedro or the CEP descriptor e.g. Td-(13)-Δ4-closo] can be used. this is determined by the complexity of the cluster. Some examples are shown below of descriptors and CEP equivalents. (The CEP descriptors are named for Casey, Evans and Powell who described the system.| number of atoms | descriptor | CEP descriptor |
|---|---|---|
| 3 | triangulo | |
| 4 | quadro | |
| 4 | tetrahedro | [Td-(13)-Δ4-closo] |
| 5 | [D3h-(131)-Δ6-closo] | |
| 6 | octahedro | [Oh-(141)-Δ8-closo] |
| 6 | triprismo | |
| 8 | antiprismo | |
| 8 | dodecahedro | [D2d-(2222)-Δ6-closo] |
| 12 | icosahedro | [Ih-(1551)-Δ20-closo] |
Examples:
decacarbonyldimanganese
10.png)
bis(pentacarbonylmanganese)(Mn—Mn)
dodecacarbonyltetrarhodium
12.png)
tri-μ-carbonyl-1:2κ2C;1:3κ2C;2:3κ2C-nonacarbonyl-
1κ2C,2κ2C,3κ2C,4κ3C-[Td-(13)-Δ4-closo]-tetrarhodium(6 Rh—Rh)
or tri-μ-carbonyl-1:2κ2C;1:3κ2C;2:3κ2C-nonacarbonyl-
1κ2C,2κ2C,3κ2C,4κ3C-tetrahedro-tetrarhodium(6 Rh—Rh)
Hydrogen names
The recommendations include a description of hydrogen names for acids. The following examples illustrate the method:- HNO3 hydrogen(nitrate)
- H2SO4 dihydrogen(sulfate)
- HSO4
− hydrogen(sulfate)(2−) - H2S dihydrogen(sulfide)
Note that the difference from the compositional naming method (hydrogen sulfide) as in hydrogen naming there is NO space between the electropositive and electronegative components.
This method gives no structural information regarding the position of the hydrons (hydrogen atoms). If this information is to be conveyed then the additive name should be used (see the list below for examples).
List of acceptable names
The recommendations give a full list of acceptable names for common acids and related anions. A selection from this list is shown below.| acid acceptable name | boric acid Boric acid Boric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a... , [B(OH)3] | dihydrogenborate, [BO(OH)2] dihydroxidooxidoborate(1—) |
hydrogenborate, [BO2(OH)]2 hydroxidodioxidoborate(2—) |
borate, [BO3]3 trioxidoborate(3—) |
|---|---|---|---|
| carbonic acid Carbonic acid Carbonic acid is the inorganic compound with the formula H2CO3 . It is also a name sometimes given to solutions of carbon dioxide in water, because such solutions contain small amounts of H2CO3. Carbonic acid forms two kinds of salts, the carbonates and the bicarbonates... , [CO(OH)2] |
hydrogencarbonate, [CO2(OH)] hydroxidodioxidocarbonate(1−) |
carbonate, [CO3]2 trioxidocarbonate(2−) |
|
| chloric acid Chloric acid Chloric acid, HClO3, is an oxoacid of chlorine, and the formal precursor of chlorate salts. It is a strong acid and oxidizing agent.... , [ClO2(OH)] hydroxidodioxidochlorine |
chlorate, [ClO3] trioxidochlorate(1−) |
||
| chlorous acid Chlorous acid Chlorous acid is an inorganic compound with the formula HClO2. It is a weak acid. Chlorine has oxidation state +3 in this acid. The pure substance is unstable, disproportionating to hypochlorous acid and chloric acid :Although the acid is difficult to obtain in pure substance, the conjugate base,... , [ClO(OH)] hydroxidooxidochlorine |
chlorite, [ClO2] dioxidochlorate(1−) |
||
| nitric acid Nitric acid Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming... , [NO2(OH)] hydroxidodioxidonitrogen |
nitrate, [NO3 trioxidonitrate(1−) |
||
| nitrous acid Nitrous acid Nitrous acid is a weak and monobasic acid known only in solution and in the form of nitrite salts.Nitrous acid is used to make diazides from amines; this occurs by nucleophilic attack of the amine onto the nitrite, reprotonation by the surrounding solvent, and double-elimination of water... ,[NO(OH)] hydroxidooxidonitrogen |
nitrite, [NO2] dioxidonitrate(1−) |
||
| perchloric acid Perchloric acid Perchloric acid is the inorganic compound with the formula HClO4. Usually encountered as an aqueous solution, this colourless compound is a strong acid comparable in strength to sulfuric and nitric acids. It is a powerful oxidizer, but its aqueous solutions up to appr. 70% are remarkably inert,... , [ClO3(OH)] hydroxidotrioxidochlorine |
perchlorate, [ClO4] tetraoxidochlorate(1−) |
||
| phosphoric acid Phosphoric acid Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way... , [PO(OH)3] trihydroxidooxidophosphorus |
dihydrogenphosphate, [PO2(OH)2] dihydroxidodioxidophosphate(1−) |
hydrogenphosphate, [PO3(OH)]2 hydroxidotrioxidophosphate(2−) |
phosphate, [PO4]3 tetraoxidophosphate(3—) |
| phosphonic acid Phosphorous acid Phosphorous acid is the compound described by the formula H3PO3. This acid is diprotic , not triprotic as might be suggested by this formula. Phosphorous acid is as an intermediate in the preparation of other phosphorus compounds.-Nomenclature and tautomerism:H3PO3 is more clearly described with... , [PHO(OH)2] hydridodihydroxidooxidophosphorus |
hydrogenphosphonate, [PHO2(OH)] hydridohydroxidodioxidophosphate(1−) |
phosphonate, [PHO3]2 hydridotrioxidophosphate(2−) |
|
| phosphorous acid, H3PO3 trihydroxidophosphorus |
dihydrogenphosphite [PO(OH)2] |
hydrogenphosphite, [PO2(OH)]2 |
phosphite, [PO3]3 trioxidophosphate(3−) |
| sulfuric acid, [SO2(OH)2] dihydroxidodioxidosulfur |
hydrogensulfate, [SO3(OH)] hydroxidotrioxidosulfate(1−) |
sulfate, [SO4]2 tetraoxidosulfate(2−) |
Solids
Stoichiometric phases are named compositionally. Non-stoichiometric phases are more difficult. Where possible formulae should be used but where necessary naming such as the following may be used:- iron(II) sulfide (iron deficient)
- molybdenum dicarbide (carbon excess)
Mineral names
Generally mineral names should not be used to specify chemical composition. However a mineral name can be used to specify the structure type in a formula e.g.- BaTiO3 (perovskite type)
Approximate formulae &variable composition
A simple notation may be used where little information on the mechanism for variability is either available or is not required to be conveyed:- ~FeS (circa or approximately)
Where there is a continuous range of composition this can be written e.g., K(Br,Cl) for a mixture of KBr and KCl and (Li2,Mg)Cl2 for a mixture of LiCl and MgCl2.
The recommendation is to use the following generalised method e.g.
- CuxNi1-x for (Cu,Ni)
- KBrxCl1-x for K(Br,Cl)
Note that cation vacancies in CoO could be described by CoO1-x
Point defects(Kröger-Vink) notation
Point defects, site symmetry and site occupancy can all be described using Kröger-Vink NotationKröger-Vink Notation
Kröger–Vink notation is set of conventions used to describe electric charge and lattice position for point defect species in crystals. It is primarily used for ionic crystals and is particularly useful for describing various defect reactions. It was proposed by F. A. Kröger and H. J. Vink.-General...
, note that the IUPAC preference is for vacancies to be specified by V rather than V (the element vanadium).
Phase nomenclature
To specify the crystal form of a compound or element the Pearson symbolPearson symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W.B. Pearson. The symbol is made up of two letters followed by a number. For example:* Diamond structure, cF8...
may be used. The use of "Strukturbericht" (e.g. A1 etc) or Greek letters is not acceptable. The Pearson symbol may be followed by the space group and the prototype formula. Examples are:
- carbon(cF 8), diamond
- RuAl(CP22, Pm3m )(CsCl type)

