Fission product yield
Encyclopedia
Nuclear fission
splits a heavy nucleus such as uranium
or plutonium
into two lighter nuclei, which are called fission products. Yield refers to the fraction of a fission product produced per fission.
Yield can be broken down by:
Isotope and element yields will change as the fission products undergo beta decay, while chain yields do not change after completion of neutron emission
by a few neutron-rich initial fission products (delayed neutron
s), with halflife measured in seconds.
A few isotopes can be produced directly by fission, but not by beta decay because the would-be precursor with atomic number one greater is stable and does not decay. Chain yields do not account for these "shadowed" isotopes; however, they have very low yields (less than a millionth as much as common fission products) because they are far less neutron-rich than the original heavy nuclei.
Yield is usually stated as percentage
per fission, so that the total yield percentages sum to 200%. Less often, it is stated as percentage of all fission products, so that the percentages sum to 100%.
 If a graph of the mass
If a graph of the mass
or mole
yield of fission products against the atomic number
of the fragments is drawn then it has two peaks, one in the area zirconium
through to palladium
and one at xenon
through to neodymium
. This is because the fission event causes the nucleus to split in an asymmetric manner.http://www.science.uwaterloo.ca/~cchieh/cact/nuctek/fissionyield.html
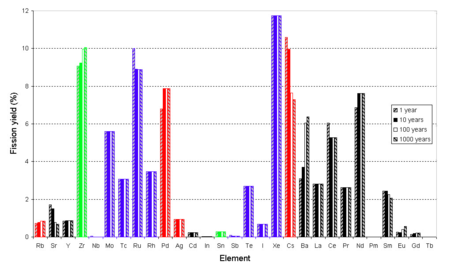
Yield vs. Z - This is a typical distribution for the fission of uranium
. Note that in the calculations used to make this graph the activation of fission products was ignored and the fission was assumed to occur in a single moment rather than a length of time. In this bar chart results are shown for different cooling times (time after fission).
Because of the stability of nuclei with even numbers of proton
s and/or neutron
s the curve of yield against element is not a smooth curve. It tends to alternate.
In general, the higher the energy of the state that undergoes nuclear fission, the more likely a symmetric fission is, hence as the neutron energy increases and/or the energy of the fissile
atom increases, the valley between the two peaks becomes more shallow; for instance, the curve of yield against mass for Pu-239 has a more shallow valley than that observed for U-235
, when the neutrons are thermal neutrons. The curves for the fission of the later actinides tend to make even more shallow valleys. In extreme cases such as 259Fm, only one peak is seen.
Yield is usually expressed relative to number of fissioning nuclei, not the number of fission product nuclei, that is, yields should sum to 200%.
The table in the next section gives yields for notable radioactive (with halflife greater than one year, plus iodine-131
) fission product
s, and (the few most absorptive) neutron poison fission products, from thermal neutron fission of U-235
(typical of nuclear power
reactors), computed from http://books.elsevier.com/companions/075067136X/pdfs/Yield.bas?mscssid=HAX80JCKT7RB8LS6F675GU2LM83N1CL6.
The yields in the table sum to only 45.5522%, including 34.8401% which have halflife greater than one year:
The remainder and the unlisted 54.4478% decay with halflife less than one year into nonradioactive nuclei.
This is before accounting for the effects of any subsequent neutron capture, e.g.:
Besides fission products, the other types of radioactive products are
Ordered by yield (thermal neutron fission of U-235

Ordered by mass number
Ordered by thermal neutron neutron absorption cross section
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
splits a heavy nucleus such as uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
or plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
into two lighter nuclei, which are called fission products. Yield refers to the fraction of a fission product produced per fission.
Yield can be broken down by:
- Individual isotopeIsotopeIsotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
- Chemical elementChemical elementA chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
spanning several isotopes of different mass numberMass numberThe mass number , also called atomic mass number or nucleon number, is the total number of protons and neutrons in an atomic nucleus. Because protons and neutrons both are baryons, the mass number A is identical with the baryon number B as of the nucleus as of the whole atom or ion...
but same atomic numberAtomic numberIn chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
. - Nuclei of a given mass numberMass numberThe mass number , also called atomic mass number or nucleon number, is the total number of protons and neutrons in an atomic nucleus. Because protons and neutrons both are baryons, the mass number A is identical with the baryon number B as of the nucleus as of the whole atom or ion...
regardless of atomic numberAtomic numberIn chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
. Known as "chain yield" because it represents a decay chainDecay chainIn nuclear science, the decay chain refers to the radioactive decay of different discrete radioactive decay products as a chained series of transformations...
of beta decayBeta decayIn nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
.
Isotope and element yields will change as the fission products undergo beta decay, while chain yields do not change after completion of neutron emission
Neutron emission
Neutron emission is a type of radioactive decay of atoms containing excess neutrons, in which a neutron is simply ejected from the nucleus. Two examples of isotopes which emit neutrons are helium-5 and beryllium-13...
by a few neutron-rich initial fission products (delayed neutron
Delayed neutron
In nuclear engineering, a delayed neutron is a neutron emitted after a nuclear fission event by one of the fission products anytime from a few milliseconds to a few minutes later....
s), with halflife measured in seconds.
A few isotopes can be produced directly by fission, but not by beta decay because the would-be precursor with atomic number one greater is stable and does not decay. Chain yields do not account for these "shadowed" isotopes; however, they have very low yields (less than a millionth as much as common fission products) because they are far less neutron-rich than the original heavy nuclei.
Yield is usually stated as percentage
Percentage
In mathematics, a percentage is a way of expressing a number as a fraction of 100 . It is often denoted using the percent sign, “%”, or the abbreviation “pct”. For example, 45% is equal to 45/100, or 0.45.Percentages are used to express how large/small one quantity is, relative to another quantity...
per fission, so that the total yield percentages sum to 200%. Less often, it is stated as percentage of all fission products, so that the percentages sum to 100%.
Mass vs. yield curve

Mass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
or mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
yield of fission products against the atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
of the fragments is drawn then it has two peaks, one in the area zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
through to palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
and one at xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
through to neodymium
Neodymium
Neodymium is a chemical element with the symbol Nd and atomic number 60. It is a soft silvery metal that tarnishes in air. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach. It is present in significant quantities in the ore minerals monazite and bastnäsite...
. This is because the fission event causes the nucleus to split in an asymmetric manner.http://www.science.uwaterloo.ca/~cchieh/cact/nuctek/fissionyield.html
Yield vs. Z - This is a typical distribution for the fission of uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
. Note that in the calculations used to make this graph the activation of fission products was ignored and the fission was assumed to occur in a single moment rather than a length of time. In this bar chart results are shown for different cooling times (time after fission).
Because of the stability of nuclei with even numbers of proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s and/or neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s the curve of yield against element is not a smooth curve. It tends to alternate.
In general, the higher the energy of the state that undergoes nuclear fission, the more likely a symmetric fission is, hence as the neutron energy increases and/or the energy of the fissile
Fissile
In nuclear engineering, a fissile material is one that is capable of sustaining a chain reaction of nuclear fission. By definition, fissile materials can sustain a chain reaction with neutrons of any energy. The predominant neutron energy may be typified by either slow neutrons or fast neutrons...
atom increases, the valley between the two peaks becomes more shallow; for instance, the curve of yield against mass for Pu-239 has a more shallow valley than that observed for U-235
Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
, when the neutrons are thermal neutrons. The curves for the fission of the later actinides tend to make even more shallow valleys. In extreme cases such as 259Fm, only one peak is seen.
Yield is usually expressed relative to number of fissioning nuclei, not the number of fission product nuclei, that is, yields should sum to 200%.
The table in the next section gives yields for notable radioactive (with halflife greater than one year, plus iodine-131
Iodine-131
Iodine-131 , also called radioiodine , is an important radioisotope of iodine. It has a radioactive decay half-life of about eight days. Its uses are mostly medical and pharmaceutical...
) fission product
Fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The...
s, and (the few most absorptive) neutron poison fission products, from thermal neutron fission of U-235
Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
(typical of nuclear power
Nuclear power
Nuclear power is the use of sustained nuclear fission to generate heat and electricity. Nuclear power plants provide about 6% of the world's energy and 13–14% of the world's electricity, with the U.S., France, and Japan together accounting for about 50% of nuclear generated electricity...
reactors), computed from http://books.elsevier.com/companions/075067136X/pdfs/Yield.bas?mscssid=HAX80JCKT7RB8LS6F675GU2LM83N1CL6.
The yields in the table sum to only 45.5522%, including 34.8401% which have halflife greater than one year:
| t½ in years | yield |
|---|---|
| 1 to 5 | 2.7252% |
| 10 to 100 | 12.5340% |
| 2 to 300,000 | 6.1251% |
| 1.5 to 16 million | 13.4494% |
The remainder and the unlisted 54.4478% decay with halflife less than one year into nonradioactive nuclei.
This is before accounting for the effects of any subsequent neutron capture, e.g.:
- 135XeXenon-135Xenon-135 is an unstable isotope of xenon with a half-life of about 9.2 hours. 135Xe is a fission product of uranium and Xe-135 is the most powerful known neutron-absorbing nuclear poison , with a significant effect on nuclear reactor operation...
capturing a neutron and becoming nonradioactive 136Xe, rather than decaying to 135Cs which is radioactive with a halflife of 2.3 million years - Nonradioactive 133Cs capturing a neutron and becoming 134Cs which is radioactive with a halflife of 2 years
- Many of the fission products with mass 147 or greater such as Promethium-147, Samarium-149, Samarium-151, Europium-155 have significant cross sections for neutron capture, so that one heavy fission product atom can undergo multiple successive neutron captures.
Besides fission products, the other types of radioactive products are
- plutoniumPlutoniumPlutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
containing 238Pu, 239Pu, 240Pu, 241Pu, and 242Pu, - minor actinidesMinor actinidesThe minor actinides are the actinide elements in used nuclear fuel other than uranium and plutonium, which are termed the major actinides. The minor actinides include neptunium, americium, curium, berkelium, californium, einsteinium, and fermium...
including 237Np, 241Am, 243AmAmericiumAmericium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
, curiumCuriumCurium is a synthetic chemical element with the symbol Cm and atomic number 96. This radioactive transuranic element of the actinide series was named after Marie Skłodowska-Curie and her husband Pierre Curie. Curium was first intentionally produced and identified in summer 1944 by the group of...
isotopes, and perhaps californiumCaliforniumCalifornium is a radioactive metallic chemical element with the symbol Cf and atomic number 98. The element was first made in the laboratory in 1950 by bombarding curium with alpha particles at the University of California, Berkeley. It is the ninth member of the actinide series and was the... - reprocessed uraniumReprocessed uraniumReprocessed uranium is the uranium recovered from nuclear reprocessing, as done commercially in France, the UK and Japan and by nuclear weapons states' military plutonium production programs. This uranium actually makes up the bulk of the material separated during reprocessing...
containing 236UUranium-236- See also :* Depleted uranium* Uranium market* Nuclear reprocessing* United States Enrichment Corporation* Nuclear fuel cycle* Nuclear power-External links:* *...
and other isotopes - tritiumTritiumTritium is a radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of protium contains one proton and no neutrons...
- activation products of neutron capture by the reactor or bomb structure or the environment
Ordered by yield (thermal neutron fission of U-235Uranium-235- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
)
| Yield | Isotope | Halflife | Comment |
|---|---|---|---|
| 6.7896% | 133Cs → Neutron capture Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled... 134Cs |
2 2.065 y | neutron capture Neutron capture Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled... (29 barns) slowly converts stable 133Cs to 134Cs, which itself is low-yield because beta decay Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... stops at 134Xe; can be further converted (140 barns) to 135Cs |
| 6.3333% | 135I → Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... 135Xe Xenon-135 Xenon-135 is an unstable isotope of xenon with a half-life of about 9.2 hours. 135Xe is a fission product of uranium and Xe-135 is the most powerful known neutron-absorbing nuclear poison , with a significant effect on nuclear reactor operation... |
6.57 h | most important neutron poison; neutron capture Neutron capture Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled... converts 10%–50% of 135Xe to 136Xe; remainder decays (9.14h) to 135Cs (2.3My) |
| 6.2956% | 93Zr | 1,530,000 1.53 My | |
| 6.1% | 99Mo | 65.94 h | Its daughter nuclide 99mTc is important in medical diagnosing. |
| 6.0899% | 137Cs | 30 30.17 y | |
| 6.0507% | 99Tc | 211,000 211 ky | Candidate for disposal by nuclear transmutation Nuclear transmutation Nuclear transmutation is the conversion of one chemical element or isotope into another. In other words, atoms of one element can be changed into atoms of other element by 'transmutation'... |
| 5.7518% | 90Sr Strontium-90 Strontium-90 is a radioactive isotope of strontium, with a half-life of 28.8 years.-Radioactivity:Natural strontium is nonradioactive and nontoxic, but 90Sr is a radioactivity hazard... |
29 28.9 y | |
| 2.8336% | 131I Iodine-131 Iodine-131 , also called radioiodine , is an important radioisotope of iodine. It has a radioactive decay half-life of about eight days. Its uses are mostly medical and pharmaceutical... |
8.02 d | |
| 2.2713% | 147Pm | 2.62 y | |
| 1.0888% | 149Sm | 100,000,000 virtually stable | 2nd most significant neutron poison |
| 0.6576% | 129I Iodine-129 Iodine-129 is long-lived radioisotope of iodine which occurs naturally, but also is of special interest in the monitoring and effects of man-made nuclear fission decay products, where it serves as both tracer and potential radiological contaminant.... |
15,700,000 15.7 My | Candidate for disposal by nuclear transmutation Nuclear transmutation Nuclear transmutation is the conversion of one chemical element or isotope into another. In other words, atoms of one element can be changed into atoms of other element by 'transmutation'... |
| 0.4203% | 151Sm | 90 90 y | neutron poison; most will be converted to stable 152Sm |
| 0.3912% | 106Ru | 1 373.6 d | |
| 0.2717% | 85Kr Krypton-85 Krypton 85 is a radioisotope of krypton.It decays into rubidium-85, with a half-life of 10.756 years and a maximum decay energy of 0.687 MeV.Its most common decay is by beta particle emission with maximum energy of 687... |
11 10.78 y | |
| 0.1629% | 107Pd | 6,500,000 6.5 My | |
| 0.0508% | 79Se Selenium-79 Selenium-79 is a radioisotope of selenium present in spent nuclear fuel and the wastes resulting from reprocessing this fuel. It is one of only 7 long-lived fission products. Its yield is low as it is near the lower end of the mass range for fission products... |
327,000 327 ky | |
| 0.0330% | 155Eu → Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... 155Gd |
5 4.76 y | both neutron poisons, most will be destroyed while fuel still in use |
| 0.0297% | 125Sb | 2.76 y | |
| 0.0236% | 126Sn | 230,000 230 ky | |
| 0.0065% | 157Gd | 100,000,000 stable | neutron poison |
| 0.0003% | 113mCd | 14 14.1 y | neutron poison, most will be destroyed while fuel still in use |

Ordered by mass numberMass numberThe mass number , also called atomic mass number or nucleon number, is the total number of protons and neutrons in an atomic nucleus. Because protons and neutrons both are baryons, the mass number A is identical with the baryon number B as of the nucleus as of the whole atom or ion...
| Yield | Isotope | ||
|---|---|---|---|
| 0.0508% | selenium-79 Selenium-79 Selenium-79 is a radioisotope of selenium present in spent nuclear fuel and the wastes resulting from reprocessing this fuel. It is one of only 7 long-lived fission products. Its yield is low as it is near the lower end of the mass range for fission products... |
||
| 0.2717% | krypton-85 Krypton-85 Krypton 85 is a radioisotope of krypton.It decays into rubidium-85, with a half-life of 10.756 years and a maximum decay energy of 0.687 MeV.Its most common decay is by beta particle emission with maximum energy of 687... |
||
| 5.7518% | strontium-90 Strontium-90 Strontium-90 is a radioactive isotope of strontium, with a half-life of 28.8 years.-Radioactivity:Natural strontium is nonradioactive and nontoxic, but 90Sr is a radioactivity hazard... |
||
| 6.2956% | zirconium-93 | ||
| 6.0507% | technetium-99 Technetium-99 Technetium-99 is an isotope of technetium which decays with a half-life of 211,000 years to stable ruthenium-99, emitting soft beta rays, but no gamma rays.... |
||
| 0.3912% | ruthenium-106 | ||
| 0.1629% | palladium-107 | ||
| 0.0003% | cadmium-113m | ||
| 0.0297% | antimony-125 | ||
| 0.0236% | tin-126 | ||
| 0.6576% | iodine-129 Iodine-129 Iodine-129 is long-lived radioisotope of iodine which occurs naturally, but also is of special interest in the monitoring and effects of man-made nuclear fission decay products, where it serves as both tracer and potential radiological contaminant.... |
||
| 2.8336% | iodine-131 Iodine-131 Iodine-131 , also called radioiodine , is an important radioisotope of iodine. It has a radioactive decay half-life of about eight days. Its uses are mostly medical and pharmaceutical... |
||
| 6.7896% | caesium-133 → Neutron capture Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled... |
caesium-134 | |
| 6.3333% | iodine-135 → Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... |
xenon-135 Xenon-135 Xenon-135 is an unstable isotope of xenon with a half-life of about 9.2 hours. 135Xe is a fission product of uranium and Xe-135 is the most powerful known neutron-absorbing nuclear poison , with a significant effect on nuclear reactor operation... → Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... |
caesium-135 |
| 6.0899% | caesium-137 Caesium-137 Caesium-137 is a radioactive isotope of caesium which is formed as a fission product by nuclear fission.It has a half-life of about 30.17 years, and decays by beta emission to a metastable nuclear isomer of barium-137: barium-137m . Caesium-137 is a radioactive isotope of caesium which is formed... |
||
| 2.2713% | promethium-147 | ||
| 1.0888% | samarium-149 | ||
| 0.4203% | samarium-151 | ||
| 0.0330% | europium-155 → Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... |
gadolinium-155 | |
| 0.0065% | gadolinium-157 |
Ordered by halflife
| Yield | Isotope | Halflife | Comment |
|---|---|---|---|
| 2.8336% | 131I Iodine-131 Iodine-131 , also called radioiodine , is an important radioisotope of iodine. It has a radioactive decay half-life of about eight days. Its uses are mostly medical and pharmaceutical... |
8.02d | Important in nuclear explosions and accidents but not in cooled spent nuclear fuel Spent nuclear fuel Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor... |
| 0.3912% | 106Ru | 373.6d | |
| 6.7896% | 133Cs → Neutron capture Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled... 134Cs |
2.065y | neutron capture Neutron capture Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled... converts a few percent of nonradioactive 133Cs to 134Cs, which has low direct yield because beta decay Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... stops at 134Xe |
| 2.2713% | 147Pm | 2.62y | |
| 0.0297% | 125Sb | 2.76y | |
| <0.0330% | 155Eu → Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... 155Gd |
4.76y | both neutron poisons, most will be destroyed by neutron capture Neutron capture Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled... while still in reactor |
| 0.2717% | 85Kr Krypton-85 Krypton 85 is a radioisotope of krypton.It decays into rubidium-85, with a half-life of 10.756 years and a maximum decay energy of 0.687 MeV.Its most common decay is by beta particle emission with maximum energy of 687... |
10.78y | Current nuclear reprocessing Nuclear reprocessing Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing... releases it to atmosphere |
| <0.0003% | 113mCd | 14.1y | most will be destroyed by neutron capture Neutron capture Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled... while still in reactor |
| 5.7518% | 90Sr Strontium-90 Strontium-90 is a radioactive isotope of strontium, with a half-life of 28.8 years.-Radioactivity:Natural strontium is nonradioactive and nontoxic, but 90Sr is a radioactivity hazard... |
28.9y | One of two principal medium-term radiation and heat sources |
| 6.0899% | 137Cs | 30.17y | One of two principal medium-term radiation and heat sources |
| <0.4203% | 151Sm | 90y | Most will be destroyed by neutron capture Neutron capture Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled... while still in reactor |
| 6.0507% | 99Tc | 211ky | Dominant radiation source among FP in period about to years; mobile in environment; candidate for disposal by nuclear transmutation Nuclear transmutation Nuclear transmutation is the conversion of one chemical element or isotope into another. In other words, atoms of one element can be changed into atoms of other element by 'transmutation'... |
| 0.0236% | 126Sn | 230ky | |
| 0.0508% | 79Se Selenium-79 Selenium-79 is a radioisotope of selenium present in spent nuclear fuel and the wastes resulting from reprocessing this fuel. It is one of only 7 long-lived fission products. Its yield is low as it is near the lower end of the mass range for fission products... |
327ky | |
| 6.2956% | 93Zr | 1.53my | |
| <6.3333% | 135Cs | 2.3my | |
| 0.1629% | 107Pd | 6.5my | |
| 0.6576% | 129I Iodine-129 Iodine-129 is long-lived radioisotope of iodine which occurs naturally, but also is of special interest in the monitoring and effects of man-made nuclear fission decay products, where it serves as both tracer and potential radiological contaminant.... |
15.7my | Mobile in environment; candidate for disposal by nuclear transmutation Nuclear transmutation Nuclear transmutation is the conversion of one chemical element or isotope into another. In other words, atoms of one element can be changed into atoms of other element by 'transmutation'... |
| <1.0888% | 149Sm | nonradioactive | neutron poison |
| <0.0065% | 157Gd | nonradioactive | neutron poison |
Ordered by thermal neutron neutron absorption cross sectionNeutron cross-sectionIn nuclear and particle physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. In conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power...
| Barns | Yield | Isotope | t½ | Comment |
|---|---|---|---|---|
| 2,650,000 | 6.3333% | 135I → Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... 135Xe Xenon-135 Xenon-135 is an unstable isotope of xenon with a half-life of about 9.2 hours. 135Xe is a fission product of uranium and Xe-135 is the most powerful known neutron-absorbing nuclear poison , with a significant effect on nuclear reactor operation... |
6.57 h | Most important neutron poison; neutron capture Neutron capture Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled... rapidly converts 135Xe to 136Xe; remainder decays (9.14 h) to 135Cs (2.3 My) |
| 254,000 | 0.0065% | 157Gd | ∞ | neutron poison, but low yield |
| 40,140 | 1.0888% | 149Sm | ∞ | 2nd most important neutron poison |
| 20,600 | 0.0003% | 113mCd | 14 14.1 y | most will be destroyed by neutron capture |
| 15,200 | 0.4203% | 151Sm | 90 90 y | most will be destroyed by neutron capture |
| 3,950 | 0.0330% | 155Eu → Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... 155Gd |
5 4.76 y | both neutron poisons |
| 96 | 2.2713% | 147Pm | 2.62 y | |
| 80 | 2.8336% | 131I Iodine-131 Iodine-131 , also called radioiodine , is an important radioisotope of iodine. It has a radioactive decay half-life of about eight days. Its uses are mostly medical and pharmaceutical... |
8.02 d | |
| 29 140 |
6.7896% | 133Cs → Neutron capture Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled... 134Cs |
∞ 2.065 y |
neutron capture Neutron capture Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled... converts a few percent of nonradioactive 133Cs to 134Cs, which has very low direct yield because beta decay Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... stops at 134Xe; further capture will add to long-lived 135Cs |
| 20 | 6.0507% | 99Tc | 211,000 211 ky | candidate for disposal by nuclear transmutation Nuclear transmutation Nuclear transmutation is the conversion of one chemical element or isotope into another. In other words, atoms of one element can be changed into atoms of other element by 'transmutation'... |
| 18 | 0.6576% | 129I Iodine-129 Iodine-129 is long-lived radioisotope of iodine which occurs naturally, but also is of special interest in the monitoring and effects of man-made nuclear fission decay products, where it serves as both tracer and potential radiological contaminant.... |
15,700,000 15.7 My | candidate for disposal by nuclear transmutation Nuclear transmutation Nuclear transmutation is the conversion of one chemical element or isotope into another. In other words, atoms of one element can be changed into atoms of other element by 'transmutation'... |
| 6.2956% | 93Zr | 1,530,000 1.53 My | transmutation impractical | |
| 0.1629% | 107Pd | 6,500,000 6.5 My | ||
| 0.2717% | 85Kr Krypton-85 Krypton 85 is a radioisotope of krypton.It decays into rubidium-85, with a half-life of 10.756 years and a maximum decay energy of 0.687 MeV.Its most common decay is by beta particle emission with maximum energy of 687... |
11 10.78 y | ||
| 5.7518% | 90Sr Strontium-90 Strontium-90 is a radioactive isotope of strontium, with a half-life of 28.8 years.-Radioactivity:Natural strontium is nonradioactive and nontoxic, but 90Sr is a radioactivity hazard... |
29 28.9 y | ||
| 0.3912% | 106Ru | 1 373.6 d | ||
| 6.0899% | 137Cs | 30 30.17 y | ||
| 0.0297% | 125Sb | 2.76 y | ||
| 0.0236% | 126Sn | 230,000 230 ky | ||
| 0.0508% | 79Se Selenium-79 Selenium-79 is a radioisotope of selenium present in spent nuclear fuel and the wastes resulting from reprocessing this fuel. It is one of only 7 long-lived fission products. Its yield is low as it is near the lower end of the mass range for fission products... |
327,000 327 ky | ||

