Decamethyldizincocene
Encyclopedia
Decamethyldizincocene is an organozinc compound
with the formula [Zn2(η5–C5Me5)2]. It is an unusual example of a compound with a Zn-Zn bond. Decamethyldizincocene is a colorless crystalline solid that burns spontaneously in the presence of oxygen and reacts with water. It is stable at room temperature and especially soluble in diethyl ether
, benzene
, pentane
, or tetrahydrofuran
.
. Among the group 12 elements, mercury
readily forms [M-M]2+ units whereas the elements cadmium
and zinc
form fewer examples of such species. Decamethyldizincocene was reported in 2004 by Carmona and coworkers as an unexpected product
of the reaction
between decamethylzincocene (Zn(C5Me5)2) and diethyl
zinc (ZnEt2). The analogous reaction of zincocene (Zn(C5H5)2) with diethyl zinc (ZnEt2) gives (η5-C5Me5)ZnEt. Therefore, the stabilizing effect of the methyl group
s on the cyclopentadienyl rings is of great importance in the formation of decamethydizincocene. The use of ZnEt2 as a reactant
is of particular significance.
The organozinc precursor is important. Diphenylzinc
(Zn(C6H5)2), despite its lower solubility, can be utilized in place of ZnEt2. On the other hand, ZnMe2 gives only the half-sandwich compound (η5-C5Me5)ZnMe.
Both (η5-C5Me5)ZnEt and decamethyldizincocene are produced from the reaction between Zn(η5-C5Me5)2 and ZnEt2. The relative amounts depend on reaction conditions, which can be optimized to favor one or the other. For instance, if this reaction is conducted in pentane
at -40 °C, (η5-C5Me5)ZnEt is the sole product. Conversely, if the reaction is conducted in diethyl ether at -10 °C, (Zn2(η5 – C5Me5)2) is the major product.
The formation of the half-sandwich complex is believed to occur via hydrocarbyl-bridged intermediates
. The reaction mechanism is, however, uncertain. Previously it was hypothesized that the creation of decamethyldizincocene occurred through the decomposition of diethylzinc, whose decomposition products would have had the capability of reducing
decamethylzincocene to decamethyldizincocene. However, it is now believed that the formation of decamethyldizincocene occurs via a radical reaction
involving the combination of two (η5-C5Me5)Zn• radicals.
In a new more efficient and more general route to decamethyldizincocene, KH is used to reduce decamethylzincocene to decamethyldizincocene (Figure 3). Other reductants
such as K
, Na
, or CaH2
may be used as well in the reduction of decamethylzincocene to decamethyldizincocene.
This complex does not react with [Lewis base]]s such PMe3, PPh3, NEt3, or pyridine
nor does it react with H2, CO2, or CO. This compound decomposes at 11 °C and sublimes at 70°C.
, and mass spectrometry
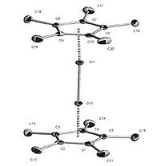
. Through X-ray diffraction methods it has been found that the zinc atoms are sandwiched between two parallel C5Me5 rings whose planes are perpendicular to the metal-metal bond axis. The separation between the two ring planes is approximately 6.40 Å. The C5Me5 rings are in an eclipsed conformation with the methyl substituents bent slightly outward (away from the central metal atoms) at angles of 3 to 6 degrees.
In mononuclear metallocenes the bending of substituent
s attached to the rings serves to prevent steric hindrance; however, the radius of a methyl group
is only 2.0 Å and therefore the bending in decamethyldizincocene does not serve this purpose since the distance between the two rings is much greater than this value. It is believed that in the case of decamethyldizincocene the bending of the methyl groups attached to the cyclopentadienyl ligands is preferential because it concentrates the electron density
away from the central, positively charged metal atoms. The separation between each Zn atom and the center of the cyclopentadienyl ring attached to it is approximately 2.04 Å and the Zn-C(ring) distances range from 2.27 to 2.30 Å. The Zn-Zn bond distance is 2.035 Å, which indicates considerably strong bonding between the two zinc atoms. This can be compared to the known [Hg-Hg]2+ bond length
of 2.5 to 2.7 Å. Two separate types of structures for dimetallocenes have been hypothesized including a coaxial structure (which is the structure of decamethyldizincocene) and a perpendicular structure in which the metal-metal bond axis is parallel to the plane of the cyclopentadienyl ligands (which is predicted to be the structure for dicuprocenes). The compound addressed in this paper is essentially linear with Zn-Zn bond angles of approximately 177°:
between the two zinc atoms, which indicates bonding. This bond has a predicted dissociation energy
of 62 kcal·mol−1 and is approximately as strong as those found among metal-halide
bonds. NBO (Natural Bond Order) analysis has indicated that sigma bond
ing occurs between the 4s orbitals of the central metal atoms with a bonding orbital
occupancy of 1.9445.9 Using fragment molecular orbital analysis
(FMOA) it has been found that there is one principal molecular orbital
that participates in the Zn-Zn bonding with approximately 88% bonding character concentrated between the Zn atoms.
Organozinc compound
Organozinc compounds in organic chemistry contain carbon to zinc chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions....
with the formula [Zn2(η5–C5Me5)2]. It is an unusual example of a compound with a Zn-Zn bond. Decamethyldizincocene is a colorless crystalline solid that burns spontaneously in the presence of oxygen and reacts with water. It is stable at room temperature and especially soluble in diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
, benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
, pentane
Pentane
Pentane is an organic compound with the formula C5H12 — that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer; the other two being called...
, or tetrahydrofuran
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
.
Synthesis
The ability of metals to form heteronuclear or homonuclear metal-metal bonds varies throughout the periodic tablePeriodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
. Among the group 12 elements, mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
readily forms [M-M]2+ units whereas the elements cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
and zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
form fewer examples of such species. Decamethyldizincocene was reported in 2004 by Carmona and coworkers as an unexpected product
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
of the reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
between decamethylzincocene (Zn(C5Me5)2) and diethyl
Ethyl group
In chemistry, an ethyl group is an alkyl substituent derived from ethane . It has the formula -C2H5 and is very often abbreviated -Et.Ethylation is the formation of a compound by introduction of the ethyl functional group, C2H5....
zinc (ZnEt2). The analogous reaction of zincocene (Zn(C5H5)2) with diethyl zinc (ZnEt2) gives (η5-C5Me5)ZnEt. Therefore, the stabilizing effect of the methyl group
Methyl group
Methyl group is a functional group derived from methane, containing one carbon atom bonded to three hydrogen atoms —CH3. The group is often abbreviated Me. Such hydrocarbon groups occur in many organic compounds. The methyl group can be found in three forms: anion, cation and radical. The anion...
s on the cyclopentadienyl rings is of great importance in the formation of decamethydizincocene. The use of ZnEt2 as a reactant
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
is of particular significance.
The organozinc precursor is important. Diphenylzinc
Diphenylzinc
Diphenylzinc is an organozinc compound. It is commonly used as the synthetic equivalent of a a Ph− synthon. Solvent-free diphenylzinc exists as dimeric PhZn2ZnPh molecules in solid state....
(Zn(C6H5)2), despite its lower solubility, can be utilized in place of ZnEt2. On the other hand, ZnMe2 gives only the half-sandwich compound (η5-C5Me5)ZnMe.
Both (η5-C5Me5)ZnEt and decamethyldizincocene are produced from the reaction between Zn(η5-C5Me5)2 and ZnEt2. The relative amounts depend on reaction conditions, which can be optimized to favor one or the other. For instance, if this reaction is conducted in pentane
Pentane
Pentane is an organic compound with the formula C5H12 — that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer; the other two being called...
at -40 °C, (η5-C5Me5)ZnEt is the sole product. Conversely, if the reaction is conducted in diethyl ether at -10 °C, (Zn2(η5 – C5Me5)2) is the major product.
Unpredictability of synthesis
The formation of decamethyldizincocene is, however, rather unpredictable. Several duplications of this reaction (under conditions that favor the formation of decamethyldizincocene) have inexplicably led to the formation of only the half-sandwich complex (η5-C5Me5)ZnEt. The formation of the products (η5-C5Me5)ZnEt and Zn2(η5 - C5Me5)2 occurs via separate, competitive reaction pathways and, therefore, the two products do not interconvert when left to react over extended periods of time.The formation of the half-sandwich complex is believed to occur via hydrocarbyl-bridged intermediates
Reaction intermediate
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete...
. The reaction mechanism is, however, uncertain. Previously it was hypothesized that the creation of decamethyldizincocene occurred through the decomposition of diethylzinc, whose decomposition products would have had the capability of reducing
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
decamethylzincocene to decamethyldizincocene. However, it is now believed that the formation of decamethyldizincocene occurs via a radical reaction
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
involving the combination of two (η5-C5Me5)Zn• radicals.
In a new more efficient and more general route to decamethyldizincocene, KH is used to reduce decamethylzincocene to decamethyldizincocene (Figure 3). Other reductants
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
such as K
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
, Na
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
, or CaH2
Calcium hydride
Calcium hydride is the chemical compound with the formula CaH2. This grey powder reacts vigorously with water liberating hydrogen gas. CaH2 is thus used as a drying agent, i.e. a desiccant....
may be used as well in the reduction of decamethylzincocene to decamethyldizincocene.
This complex does not react with [Lewis base]]s such PMe3, PPh3, NEt3, or pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
nor does it react with H2, CO2, or CO. This compound decomposes at 11 °C and sublimes at 70°C.
Structure
Various methods have been employed in order to determine the structure of decamethyldizincocene, including x-ray diffraction, 1H NMRNMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
, and mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
. Through X-ray diffraction methods it has been found that the zinc atoms are sandwiched between two parallel C5Me5 rings whose planes are perpendicular to the metal-metal bond axis. The separation between the two ring planes is approximately 6.40 Å. The C5Me5 rings are in an eclipsed conformation with the methyl substituents bent slightly outward (away from the central metal atoms) at angles of 3 to 6 degrees.
In mononuclear metallocenes the bending of substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s attached to the rings serves to prevent steric hindrance; however, the radius of a methyl group
Methyl group
Methyl group is a functional group derived from methane, containing one carbon atom bonded to three hydrogen atoms —CH3. The group is often abbreviated Me. Such hydrocarbon groups occur in many organic compounds. The methyl group can be found in three forms: anion, cation and radical. The anion...
is only 2.0 Å and therefore the bending in decamethyldizincocene does not serve this purpose since the distance between the two rings is much greater than this value. It is believed that in the case of decamethyldizincocene the bending of the methyl groups attached to the cyclopentadienyl ligands is preferential because it concentrates the electron density
Electron density
Electron density is the measure of the probability of an electron being present at a specific location.In molecules, regions of electron density are usually found around the atom, and its bonds...
away from the central, positively charged metal atoms. The separation between each Zn atom and the center of the cyclopentadienyl ring attached to it is approximately 2.04 Å and the Zn-C(ring) distances range from 2.27 to 2.30 Å. The Zn-Zn bond distance is 2.035 Å, which indicates considerably strong bonding between the two zinc atoms. This can be compared to the known [Hg-Hg]2+ bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
of 2.5 to 2.7 Å. Two separate types of structures for dimetallocenes have been hypothesized including a coaxial structure (which is the structure of decamethyldizincocene) and a perpendicular structure in which the metal-metal bond axis is parallel to the plane of the cyclopentadienyl ligands (which is predicted to be the structure for dicuprocenes). The compound addressed in this paper is essentially linear with Zn-Zn bond angles of approximately 177°:
Absence of bridging ligands
1H NMR and mass spectrometry studies have been useful in proving that decamethyldizincocene does not include bridging ligands. This study is important considering that the complex previously hypothesized to be Co2(η5-C5Me5)2 was later found using 1H NMR and mass spectrometry data to be supported by three bridging hydrogens. The 1H NMR of decamethyldizincocene shows only one signal at δ 2.02 due to the hydrogens attached to the methyl groups on the cyclopentadienyl ligands.Electronic structure and bonding characteristics
Decamethyldizincocene has an accumulation of electron densityElectron density
Electron density is the measure of the probability of an electron being present at a specific location.In molecules, regions of electron density are usually found around the atom, and its bonds...
between the two zinc atoms, which indicates bonding. This bond has a predicted dissociation energy
Bond dissociation energy
In chemistry, bond-dissociation energy or D0, is one measure of the bond strength in a chemical bond. It is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K...
of 62 kcal·mol−1 and is approximately as strong as those found among metal-halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
bonds. NBO (Natural Bond Order) analysis has indicated that sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
ing occurs between the 4s orbitals of the central metal atoms with a bonding orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
occupancy of 1.9445.9 Using fragment molecular orbital analysis
Fragment Molecular Orbital
The fragment molecular orbital method is a computational method that can compute very large molecular systems with thousands of atoms using ab initio quantum-chemical wave functions.- History of FMO and related methods :...
(FMOA) it has been found that there is one principal molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
that participates in the Zn-Zn bonding with approximately 88% bonding character concentrated between the Zn atoms.

