
Coulometry
Encyclopedia
Coulometry is the name given to a group of techniques in analytical chemistry
that determine the amount of matter transformed during an electrolysis
reaction by measuring the amount of electricity
(in coulombs) consumed or produced.
There are two basic categories of coulometric techniques. Potentiostatic coulometry involves holding the electric potential
constant during the reaction using a potentiostat
. The other, called coulometric titration or amperostatic coulometry, keeps the current (measured in amperes) constant using an amperostat
.
is a technique most commonly referred to as "bulk electrolysis
". The working electrode
is kept at a constant potential and the current which flows through the circuit is measured. This constant potential is applied long enough to fully reduce or oxidize all of the substrate in a given solution. As the substrate is consumed, the current also decreases, approaching zero when the conversion is complete. The sample mass, molecular mass, number of electrons in the electrode reaction, and number of electrons passed during the experiment are all related by Faraday's laws
. It follows that, if three of the values are known, then the fourth can be calculated.
Bulk electrolysis is often used to unambiguously assign the number of electrons consumed in a reaction observed through voltammetry
. It also has the added benefit of producing a solution of a species (oxidation state) which may not be accessible through chemical routes. This species can then be isolated or further characterized while in solution.
The rate of such reactions is not determined by the concentration of the solution, but rather the mass transfer
of the substrate in the solution to the electrode surface. Rates will increase when the volume of the solution is decreased, the solution is stirred more rapidly, or the area of the working electrode is increased. Since mass transfer is so important the solution is stirred during a bulk electrolysis. However, this technique is generally not considered a hydrodynamic technique
, since a laminar flow of solution against the electrode is neither the objective nor outcome of the stirring.
The extent to which a reaction goes to completion is also related to how much greater the applied potential is than the reduction potential of interest. In the case where multiple reduction potentials are of interest, it is often difficult to set an electrolysis potential a "safe" distance (such as 200 mV) past a redox event. The result is incomplete conversion of the substrate, or else conversion of some of the substrate to the more reduced form. This factor must be considered when analyzing the current passed and when attempting to do further analysis/isolation/experiments with the substrate solution.
An advantage to this kind of analysis over electrogravimetry
is that it does not require that the product of the reaction be weighed. This is useful for reactions where the product does not deposit as a solid, such as the determination of the amount of arsenic
in a sample from the electrolysis of arsenous acid
(H3AsO3) to arsenic acid
(H3AsO4).
shifts dramatically. This potential shift indicates the endpoint. The magnitude of the current (in ampere
s) and the duration of the current (second
s) can be used to determine the mole
s of the unknown species in solution. When the volume of the solution is known, then the molarity of the unknown species can be determined.
uses a coulometric titration to determine the amount of water in a sample. It can determine concentrations of water on the order of milligrams per liter. It is used to find the amount of water in substances such as butter
, sugar
, cheese
, paper
, and petroleum
.
The reaction involves converting solid iodine into hydrogen iodide in the presence of sulfur dioxide and water. Methanol
is most often used as the solvent, but ethylene glycol
and diethylene glycol
also work. Pyridine
is often used to prevent the build up of sulfuric acid
, although the use of imidazole
and diethanolamine for this role are becoming more common. All reagents must be anhydrous
for the analysis to be quantitative. The balanced chemical equation, using methanol and pyridine, is:
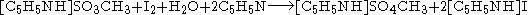
In this reaction, a single molecule of water reacts with a molecule of iodine. Since this technique is used to determine the water content of samples, atmospheric humidity could alter the results. Therefore, the system is usually isolated with drying tubes or placed in an inert gas
container. In addition, the solvent will undoubtedly have some water in it so the solvent’s water content must be measured to compensate for this inaccuracy.
To determine the amount of water in the sample, analysis must first be performed using either back or direct titration
. In the direct method, just enough of the reagents will be added to completely use up all of the water. At this point in the titration, the current approaches zero. It is then possible to relate the amount of reagents used to the amount of water in the system via stoichiometry
. The back-titration method is similar, but involves the addition of an excess of the reagent. This excess is then consumed by adding a known amount of a standard solution with known water content. The result reflects the water content of the sample and the standard solution. Since the amount of water in the standard solution is known, the difference reflects the water content of the sample.
needed to dissolve a well-defined area of the coating. The film thickness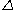 is proportional to the constant current
is proportional to the constant current 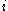 , the molecular weight
, the molecular weight 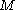 of the metal, the density
of the metal, the density
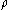 of the metal, and the surface area
of the metal, and the surface area 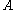 :
:
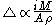
The electrodes for this reaction are often platinum electrode and an electrode that relates to the reaction. For tin coating on a copper wire, a tin electrode is used, while a sodium chloride-zinc sulfate electrode would be used to determine the zinc film on a piece of steel. Special cells have been created to adhere to the surface of the metal to measure its thickness. These are basically columns with the internal electrodes with magnets or weights to attach to the surface. The results obtained by this coulometric method are similar to those achieved by other chemical and metallurgic techniques.
in the "integrator"-type circuit. The current passed through the resistor R1 makes a potential drop which is integrated by operational amplifier on the capacitor
plates; the higher current, the larger the potential drop. The current need not be constant. In such scheme Vout is proportional of the passed charge (i*t). Sensitivity of the coulometer can be changed by choosing of the appropriate value of R1.
"Voltameter" is a synonym for "coulometer".
Electroanalytical method
Electroanalytical methods are a class of techniques in analytical chemistry which study an analyte by measuring the potential and/or current in an electrochemical cell containing the analyte. These methods can be broken down into several categories depending on which aspects of the cell are...
that determine the amount of matter transformed during an electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
reaction by measuring the amount of electricity
Electricity
Electricity is a general term encompassing a variety of phenomena resulting from the presence and flow of electric charge. These include many easily recognizable phenomena, such as lightning, static electricity, and the flow of electrical current in an electrical wire...
(in coulombs) consumed or produced.
There are two basic categories of coulometric techniques. Potentiostatic coulometry involves holding the electric potential
Electric potential
In classical electromagnetism, the electric potential at a point within a defined space is equal to the electric potential energy at that location divided by the charge there...
constant during the reaction using a potentiostat
Potentiostat
A potentiostat is the electronic hardware required to control a three electrode cell and run most electroanalytical experiments. A Bipotentiostat and polypotentiostat are potentiostats capable of controlling two working electrodes and more than two working electrodes, respectively.The system...
. The other, called coulometric titration or amperostatic coulometry, keeps the current (measured in amperes) constant using an amperostat
Amperostat
An amperostat is a control and measuring device used to keep constant the current flowing though electrolytic cells in coulometric titrations, disregarding changes in the load itself. Synonym is "galvanostat"...
.
Potentiostatic coulometry
Potentiostatic coulometryBulk electrolysis
Bulk electrolysis is also known as potentiostatic coulometry or controlled potential coulometry. The experiment is a form of coulometry which generally employees a three electrode system controlled by a potentiostat. In the experiment the working electrode is held at a constant potential and...
is a technique most commonly referred to as "bulk electrolysis
Bulk electrolysis
Bulk electrolysis is also known as potentiostatic coulometry or controlled potential coulometry. The experiment is a form of coulometry which generally employees a three electrode system controlled by a potentiostat. In the experiment the working electrode is held at a constant potential and...
". The working electrode
Working electrode
The working electrode is the electrode in an electrochemical system on which the reaction of interest is occurring. The working electrode is often used in conjunction with an auxiliary electrode, and a reference electrode in a three electrode system...
is kept at a constant potential and the current which flows through the circuit is measured. This constant potential is applied long enough to fully reduce or oxidize all of the substrate in a given solution. As the substrate is consumed, the current also decreases, approaching zero when the conversion is complete. The sample mass, molecular mass, number of electrons in the electrode reaction, and number of electrons passed during the experiment are all related by Faraday's laws
Faraday's laws of electrolysis
Faraday's laws of electrolysis are quantitative relationships based on the electrochemical researches published by Michael Faraday in 1834.-Statements of the laws:Several versions of the laws can be found in textbooks and the scientific literature...
. It follows that, if three of the values are known, then the fourth can be calculated.
Bulk electrolysis is often used to unambiguously assign the number of electrons consumed in a reaction observed through voltammetry
Voltammetry
Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied.- Three electrode system :...
. It also has the added benefit of producing a solution of a species (oxidation state) which may not be accessible through chemical routes. This species can then be isolated or further characterized while in solution.
The rate of such reactions is not determined by the concentration of the solution, but rather the mass transfer
Mass transfer
Mass transfer is the net movement of mass from one location, usually meaning a stream, phase, fraction or component, to another. Mass transfer occurs in many processes, such as absorption, evaporation, adsorption, drying, precipitation, membrane filtration, and distillation. Mass transfer is used...
of the substrate in the solution to the electrode surface. Rates will increase when the volume of the solution is decreased, the solution is stirred more rapidly, or the area of the working electrode is increased. Since mass transfer is so important the solution is stirred during a bulk electrolysis. However, this technique is generally not considered a hydrodynamic technique
Hydrodynamic technique
Hydrodynamic technique is a subcategory of electroanalytical methods in which the analyte solution flows relative to a working electrode. In many voltammetry techniques, the solution is intentionally left still to allow diffusion controlled mass transfer...
, since a laminar flow of solution against the electrode is neither the objective nor outcome of the stirring.
The extent to which a reaction goes to completion is also related to how much greater the applied potential is than the reduction potential of interest. In the case where multiple reduction potentials are of interest, it is often difficult to set an electrolysis potential a "safe" distance (such as 200 mV) past a redox event. The result is incomplete conversion of the substrate, or else conversion of some of the substrate to the more reduced form. This factor must be considered when analyzing the current passed and when attempting to do further analysis/isolation/experiments with the substrate solution.
An advantage to this kind of analysis over electrogravimetry
Electrogravimetry
Electrogravimetry is a method used to separate and quantify ions of a substance, usually a metal. In this process, the analyte solution is electrolyzed. Electrochemical reduction causes the analyte to be deposited on the cathode...
is that it does not require that the product of the reaction be weighed. This is useful for reactions where the product does not deposit as a solid, such as the determination of the amount of arsenic
Arsenic
Arsenic is a chemical element with the symbol As, atomic number 33 and relative atomic mass 74.92. Arsenic occurs in many minerals, usually in conjunction with sulfur and metals, and also as a pure elemental crystal. It was first documented by Albertus Magnus in 1250.Arsenic is a metalloid...
in a sample from the electrolysis of arsenous acid
Arsenous acid
Arsenous acid, also known as arsenious acid, is the inorganic compound with the formula H3AsO3. It is known to occur in aqueous solutions, but it has not been isolated as a pure material, although this fact does not detract from the significance of As3.-Properties:As3 is a pyramidal molecule...
(H3AsO3) to arsenic acid
Arsenic acid
Arsenic acid is the chemical compound with the formula H3AsO4. More descriptively written as AsO3, this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as such has not been isolated, but only found in solution where it...
(H3AsO4).
Coulometric titration
Coulometric titrations use a constant current system to accurately quantify the concentration of a species. In this experiment, the applied current is equivalent to a titrant. Current is applied to the unknown solution until all of the unknown species is either oxidized or reduced to a new state, at which point the potential of the working electrodeWorking electrode
The working electrode is the electrode in an electrochemical system on which the reaction of interest is occurring. The working electrode is often used in conjunction with an auxiliary electrode, and a reference electrode in a three electrode system...
shifts dramatically. This potential shift indicates the endpoint. The magnitude of the current (in ampere
Ampere
The ampere , often shortened to amp, is the SI unit of electric current and is one of the seven SI base units. It is named after André-Marie Ampère , French mathematician and physicist, considered the father of electrodynamics...
s) and the duration of the current (second
Second
The second is a unit of measurement of time, and is the International System of Units base unit of time. It may be measured using a clock....
s) can be used to determine the mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
s of the unknown species in solution. When the volume of the solution is known, then the molarity of the unknown species can be determined.
Karl Fischer reaction
The Karl Fischer reactionKarl Fischer titration
Karl Fischer titration is a classic titration method in analytical chemistry that uses coulometric or volumetric titration to determine trace amounts of water in a sample. It was invented in 1935 by the German chemist Karl Fischer.-Coulometric titration:...
uses a coulometric titration to determine the amount of water in a sample. It can determine concentrations of water on the order of milligrams per liter. It is used to find the amount of water in substances such as butter
Butter
Butter is a dairy product made by churning fresh or fermented cream or milk. It is generally used as a spread and a condiment, as well as in cooking applications, such as baking, sauce making, and pan frying...
, sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
, cheese
Cheese
Cheese is a generic term for a diverse group of milk-based food products. Cheese is produced throughout the world in wide-ranging flavors, textures, and forms....
, paper
Paper
Paper is a thin material mainly used for writing upon, printing upon, drawing or for packaging. It is produced by pressing together moist fibers, typically cellulose pulp derived from wood, rags or grasses, and drying them into flexible sheets....
, and petroleum
Petroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
.
The reaction involves converting solid iodine into hydrogen iodide in the presence of sulfur dioxide and water. Methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
is most often used as the solvent, but ethylene glycol
Ethylene glycol
Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
and diethylene glycol
Diethylene glycol
Diethylene glycol is an organic compound with the formula 2O. It is a colorless, practically odorless, poisonous, and hygroscopic liquid with a sweetish taste. It is miscible in water, alcohol, ether, acetone, and ethylene glycol. DEG is a widely used solvent...
also work. Pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
is often used to prevent the build up of sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
, although the use of imidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
and diethanolamine for this role are becoming more common. All reagents must be anhydrous
Anhydrous
As a general term, a substance is said to be anhydrous if it contains no water. The way of achieving the anhydrous form differs from one substance to another...
for the analysis to be quantitative. The balanced chemical equation, using methanol and pyridine, is:
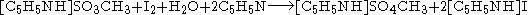
In this reaction, a single molecule of water reacts with a molecule of iodine. Since this technique is used to determine the water content of samples, atmospheric humidity could alter the results. Therefore, the system is usually isolated with drying tubes or placed in an inert gas
Inert gas
An inert gas is a non-reactive gas used during chemical synthesis, chemical analysis, or preservation of reactive materials. Inert gases are selected for specific settings for which they are functionally inert since the cost of the gas and the cost of purifying the gas are usually a consideration...
container. In addition, the solvent will undoubtedly have some water in it so the solvent’s water content must be measured to compensate for this inaccuracy.
To determine the amount of water in the sample, analysis must first be performed using either back or direct titration
Titration
Titration, also known as titrimetry, is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte. Because volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the...
. In the direct method, just enough of the reagents will be added to completely use up all of the water. At this point in the titration, the current approaches zero. It is then possible to relate the amount of reagents used to the amount of water in the system via stoichiometry
Stoichiometry
Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
. The back-titration method is similar, but involves the addition of an excess of the reagent. This excess is then consumed by adding a known amount of a standard solution with known water content. The result reflects the water content of the sample and the standard solution. Since the amount of water in the standard solution is known, the difference reflects the water content of the sample.
Determination of film thickness
Coulometry can be used in the determination of the thickness of metallic coatings. This is performed by measuring the quantity of electricityQuantity of electricity
In physics the term quantity of electricity refers to the quantity of electric charge. It is designated by the letter Q and in the SI system is measured in derived units called Coulombs.- Pre-English origins :...
needed to dissolve a well-defined area of the coating. The film thickness
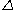 is proportional to the constant current
is proportional to the constant current 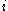 , the molecular weight
, the molecular weight 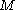 of the metal, the density
of the metal, the densityDensity
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
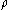 of the metal, and the surface area
of the metal, and the surface area 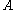 :
: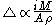
The electrodes for this reaction are often platinum electrode and an electrode that relates to the reaction. For tin coating on a copper wire, a tin electrode is used, while a sodium chloride-zinc sulfate electrode would be used to determine the zinc film on a piece of steel. Special cells have been created to adhere to the surface of the metal to measure its thickness. These are basically columns with the internal electrodes with magnets or weights to attach to the surface. The results obtained by this coulometric method are similar to those achieved by other chemical and metallurgic techniques.
Electronic coulometer
The electronic coulometer is based on the application of the operational amplifierOperational amplifier
An operational amplifier is a DC-coupled high-gain electronic voltage amplifier with a differential input and, usually, a single-ended output...
in the "integrator"-type circuit. The current passed through the resistor R1 makes a potential drop which is integrated by operational amplifier on the capacitor
Capacitor
A capacitor is a passive two-terminal electrical component used to store energy in an electric field. The forms of practical capacitors vary widely, but all contain at least two electrical conductors separated by a dielectric ; for example, one common construction consists of metal foils separated...
plates; the higher current, the larger the potential drop. The current need not be constant. In such scheme Vout is proportional of the passed charge (i*t). Sensitivity of the coulometer can be changed by choosing of the appropriate value of R1.
Electrochemical coulometers
There are three common types of coulometers based on electrochemical processes:- Copper coulometerCopper coulometerThe copper coulometer is a one of the common application of the copper-copper sulfate electrode. Such a coulometer consists of two identical copper electrodes immersed in slightly acidic pH-buffered solution of copper sulfate. Passing of current through the element leads to the anodic dissolution...
- Mercury coulometerMercury coulometerMercury coulometer is an electroanalytical chemistry device using mercury to determine the amount of matter transformed during the following reaction:...
- Hofmann voltameterHofmann voltameterA Hofmann voltameter is an apparatus for electrolyzing water, invented by August Wilhelm von Hofmann in 1866. It consists of three joined upright cylinders, usually glass. The inner cylinder is open at the top to allow addition of water and an ionic compound to improve conductivity, such as a...
"Voltameter" is a synonym for "coulometer".

