
Copper coulometer
Encyclopedia
The copper coulometer is a one of the common application of the copper-copper(II) sulfate electrode
. Such a coulometer
consists of two identical copper electrodes immersed in slightly acidic pH-buffered
solution of copper(II) sulfate. Passing of current through the element leads to the anodic dissolution
of the metal on anode
and simultaneous deposition
of copper
-ions on the cathode
. These reactions have 100% efficiency over a wide range of the current densities.
passed through the cell can be easily calculated by mass changes of any of the electrodes: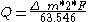 ,
,
where:
Although from a theoretical and historical point of view this apparatus is interesting, present day electronic measurement of time and electrical current provide in their multiplication the amount of passed coulombs much easier, with greater precission, and in a shorter period of time than is possible by weighing the electrodes.
Copper-copper(II) sulfate electrode
The Copper-copper sulfate electrode is a reference electrode of the first kind, based on the redox reaction with participation of the metal and its salt - copper sulfate....
. Such a coulometer
Coulometry
Coulometry is the name given to a group of techniques in analytical chemistry that determine the amount of matter transformed during an electrolysis reaction by measuring the amount of electricity consumed or produced....
consists of two identical copper electrodes immersed in slightly acidic pH-buffered
Buffer solution
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
solution of copper(II) sulfate. Passing of current through the element leads to the anodic dissolution
Solvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
of the metal on anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
and simultaneous deposition
Deposition (chemistry)
In chemistry, deposition is the settling of particles or sediment from a solution, suspension and mixture or vapor onto a pre-existing surface...
of copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
-ions on the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
. These reactions have 100% efficiency over a wide range of the current densities.
Calculation
Amount of the quantity of electricityQuantity of electricity
In physics the term quantity of electricity refers to the quantity of electric charge. It is designated by the letter Q and in the SI system is measured in derived units called Coulombs.- Pre-English origins :...
passed through the cell can be easily calculated by mass changes of any of the electrodes:
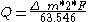 ,
,where:
- Q is the quantity of electricity (coulombs)
-
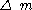 is the mass transported (gm)
is the mass transported (gm) - 63.546 is the atomic weightAtomic weightAtomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
of copper (the factor 2 is due to the transport of divalentDivalentIn chemistry, a divalent ion or molecule has a valence of two and thus can form two bonds with other ions or molecules. An older term for divalent is bivalent....
ions) - F is the Faraday constant (96485.3383)
Although from a theoretical and historical point of view this apparatus is interesting, present day electronic measurement of time and electrical current provide in their multiplication the amount of passed coulombs much easier, with greater precission, and in a shorter period of time than is possible by weighing the electrodes.

