
Colors of chemicals
Encyclopedia
The color of chemicals is a physical property
of chemicals that in most cases comes from the excitation of electrons
due to an absorption of energy performed by the chemical. What is seen by the eye is not the color absorbed, but the complementary color
from the removal of the absorbed wavelength
s.
The study of chemical structure by means of energy absorption and release is generally referred to as spectroscopy
.
 All atoms and molecules are capable of absorbing and releasing energy in the form of photon
All atoms and molecules are capable of absorbing and releasing energy in the form of photon
s, accompanied by a change of quantum state. The amount of energy absorbed or released is the difference between the energies of the two quantum states. There are various types of quantum state, including, for example, the rotational and vibrational states of a molecule. However the release of energy visible to the human eye, commonly referred to as visible light, spans the wavelengths approximately 380 nm to 760 nm, depending on the individual, and photons in this range usually accompany a change in atomic
or molecular orbital
quantum state. The perception of light is governed by three types of color
receptors in the eye, which are sensitive to different ranges of wavelength within this band.
The relationship between energy and wavelength is determined by the equation: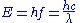
where E is the energy of the quantum
(photon
), f is the frequency
of the light wave, h is Planck's constant, is the wavelength and c is the speed of light
is the wavelength and c is the speed of light
.
The relationships between the energies of the various quantum states are treated by atomic orbital
, molecular orbital
, and Ligand Field Theory
. If photons of a particular wavelength are absorbed by matter, then when we observe light reflected from or transmitted through that matter, what we see is the complementary color
, made up of the other visible wavelengths remaining. For example beta-carotene
has maximum absorption at 454 nm (blue light), consequently what visible light remains appears orange.
and RGB
color wheel
s rather than the traditional RYB
color wheel.
This can only be used as a very rough guide, for instance if a narrow range of wavelengths within the band 647-700 is absorbed, then the blue and green receptors will be fully stimulated, making cyan, and the red receptor will be partially stimulated, diluting the cyan to a greyish hue.
) and organic compounds (e.g. ethanol) are colorless. Transition metal
compounds are often colored because of transitions of electrons between d-orbitals of different energy. (see Transition metal#Coloured compounds). Organic compounds tend to be colored when there is extensive conjugation
, causing the energy gap between the HOMO and LUMO
to decrease, bringing the absorption band from the UV to the visible region. Similarly, color is due to the energy absorbed by the compound, when an electron transitions from the HOMO to the LUMO. Lycopene
is a classic example of a compound with extensive conjugation (11 conjugated double bonds), giving rise to an intense red color. Charge-transfer complexes tend to have very intense colors for different reasons.
It is important to note, however, that elemental colors will vary depending on what they are complexed with, often as well as their chemical state. An example with vanadium(III); VCl3 has a distinctive reddish hue, whilst V2O3 appears black.
Cobalt chloride is pink or blue depending on the state of hydration (blue dry, pink with water) so it's used as a moisture indicator in silica gel. Zinc Oxide is white, but at higher temperatures becomes yellow, returning to white as it cools.
) (see also Flame test
)
. The loop with the adhered powders is then heated in a flame until it fusses and the color of the resulting bead observed.
Physical property
A physical property is any property that is measurable whose value describes a physical system's state. The changes in the physical properties of a system can be used to describe its transformations ....
of chemicals that in most cases comes from the excitation of electrons
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
due to an absorption of energy performed by the chemical. What is seen by the eye is not the color absorbed, but the complementary color
Complementary color
Complementary colors are pairs of colors that are of “opposite” hue in some color model. The exact hue “complementary” to a given hue depends on the model in question, and perceptually uniform, additive, and subtractive color models, for example, have differing complements for any given color.-...
from the removal of the absorbed wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
s.
The study of chemical structure by means of energy absorption and release is generally referred to as spectroscopy
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
.
Theory

Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s, accompanied by a change of quantum state. The amount of energy absorbed or released is the difference between the energies of the two quantum states. There are various types of quantum state, including, for example, the rotational and vibrational states of a molecule. However the release of energy visible to the human eye, commonly referred to as visible light, spans the wavelengths approximately 380 nm to 760 nm, depending on the individual, and photons in this range usually accompany a change in atomic
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
or molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
quantum state. The perception of light is governed by three types of color
Color
Color or colour is the visual perceptual property corresponding in humans to the categories called red, green, blue and others. Color derives from the spectrum of light interacting in the eye with the spectral sensitivities of the light receptors...
receptors in the eye, which are sensitive to different ranges of wavelength within this band.
The relationship between energy and wavelength is determined by the equation:
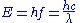
where E is the energy of the quantum
Quantum
In physics, a quantum is the minimum amount of any physical entity involved in an interaction. Behind this, one finds the fundamental notion that a physical property may be "quantized," referred to as "the hypothesis of quantization". This means that the magnitude can take on only certain discrete...
(photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
), f is the frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
of the light wave, h is Planck's constant,
 is the wavelength and c is the speed of light
is the wavelength and c is the speed of lightSpeed of light
The speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
.
The relationships between the energies of the various quantum states are treated by atomic orbital
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
, molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
, and Ligand Field Theory
Ligand field theory
Ligand field theory describes the bonding, orbital arrangement, and other characteristics of coordination complexes. It represents an application of molecular orbital theory to transition metal complexes. A transition metal ion has nine valence atomic orbitals, five d, one s, and three p orbitals...
. If photons of a particular wavelength are absorbed by matter, then when we observe light reflected from or transmitted through that matter, what we see is the complementary color
Complementary color
Complementary colors are pairs of colors that are of “opposite” hue in some color model. The exact hue “complementary” to a given hue depends on the model in question, and perceptually uniform, additive, and subtractive color models, for example, have differing complements for any given color.-...
, made up of the other visible wavelengths remaining. For example beta-carotene
Beta-carotene
β-Carotene is a strongly-coloured red-orange pigment abundant in plants and fruits. It is an organic compound and chemically is classified as a hydrocarbon and specifically as a terpenoid , reflecting its derivation from isoprene units...
has maximum absorption at 454 nm (blue light), consequently what visible light remains appears orange.
Colors by wavelength
Below is a rough table of wavelengths, colors and complementary colors. This utilizes the scientific CMYCMYK color model
The CMYK color model is a subtractive color model, used in color printing, and is also used to describe the printing process itself. CMYK refers to the four inks used in some color printing: cyan, magenta, yellow, and key...
and RGB
RGB color model
The RGB color model is an additive color model in which red, green, and blue light is added together in various ways to reproduce a broad array of colors...
color wheel
Color wheel
A color wheel or color circle is an abstract illustrative organization of color hues around a circle that shows relationships between primary colors, secondary colors, complementary colors, etc....
s rather than the traditional RYB
RYB color model
RYB is a historical set of colors used in subtractive color mixing, and is one commonly used set of primary colors...
color wheel.
| Wavelength (nm) | Color | Complementary Color |
|---|---|---|
| 400-424 | Violet | Green-yellow |
| 424-491 | Blue | Yellow |
| 491-570 | Green | Violet |
| 570-585 | Yellow | Blue |
| 585-647 | Orange | Cyan-Blue |
| 647-700 | Red | Cyan |
This can only be used as a very rough guide, for instance if a narrow range of wavelengths within the band 647-700 is absorbed, then the blue and green receptors will be fully stimulated, making cyan, and the red receptor will be partially stimulated, diluting the cyan to a greyish hue.
By category
The vast majority of simple inorganic (e.g. sodium chlorideSodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
) and organic compounds (e.g. ethanol) are colorless. Transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
compounds are often colored because of transitions of electrons between d-orbitals of different energy. (see Transition metal#Coloured compounds). Organic compounds tend to be colored when there is extensive conjugation
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
, causing the energy gap between the HOMO and LUMO
HOMO/LUMO
HOMO and LUMO are acronyms for highest occupied molecular orbital and lowest unoccupied molecular orbital, respectively. The energy difference between the HOMO and LUMO is termed the HOMO-LUMO gap...
to decrease, bringing the absorption band from the UV to the visible region. Similarly, color is due to the energy absorbed by the compound, when an electron transitions from the HOMO to the LUMO. Lycopene
Lycopene
Lycopene is a bright red carotene and carotenoid pigment and phytochemical found in tomatoes and other red fruits and vegetables, such as red carrots, watermelons and papayas...
is a classic example of a compound with extensive conjugation (11 conjugated double bonds), giving rise to an intense red color. Charge-transfer complexes tend to have very intense colors for different reasons.
Ions in aqueous solution
| Name | Formula | Color |
|---|---|---|
| Alkali metals | M+ | None |
| Alkaline earth metals | M2+ | None |
| Scandium(III) | Sc3+ | None |
| Titanium(III) | Ti3+ | Violet |
| Titanyl | TiO2+ | None |
| Vanadium(II) | V2+ | Lavender |
| Vanadium(III) | V3+ | Dark grey/green |
| Vanadyl | VO2+ | Blue |
| Pervanadyl | VO2+ | Yellow |
| Metavanadate | VO3- | None |
| Orthovanadate | VO43- | None |
| Chromium(III) | Cr3+ | Blue-green |
| Chromate | CrO4 2- | Colorless or Yellow(sometimes) |
| Dichromate | Cr2O72- | Orange |
| Manganese(II) | Mn2+ | Colourless |
| Manganate(VII) (Permanganate) | MnO4- | Deep violet |
| Manganate(VI) | MnO42- | Dark green |
| Manganate(V) | MnO43- | Deep blue |
| Iron(II) | Fe2+ | Light blue |
| Iron(III) | Fe3+ | Yellow/brown |
| Cobalt(II) | Co2+ | Pink |
| Cobalt-ammonium complex | Co(NH3)63+ | Yellow/orange |
| Nickel(II) | Ni2+ | Light green |
| Nickel-ammonium complex | Ni(NH3)62+ | Lavender/blue |
| Copper(II) | Cu 2+ | Blue |
| Copper-ammonium complex | Cu(NH3)42+ | Royal Blue |
| Tetrachloro-copper complex | CuCl42- | Yellow/green |
| Zinc(II) | Zn2+ | Bluish-white |
| Silver | Ag+ | None |
It is important to note, however, that elemental colors will vary depending on what they are complexed with, often as well as their chemical state. An example with vanadium(III); VCl3 has a distinctive reddish hue, whilst V2O3 appears black.
Salts
Predicting the color of a compound can be extremely complicated. Some examples include:Cobalt chloride is pink or blue depending on the state of hydration (blue dry, pink with water) so it's used as a moisture indicator in silica gel. Zinc Oxide is white, but at higher temperatures becomes yellow, returning to white as it cools.
| Name | Formula | Color | Picture |
|---|---|---|---|
| Copper(II) sulfate | CuSO4 | White | |
| Copper(II) sulfate pentahydrate | CuSO4 · 5H2O | Blue |  |
| Cobalt(II) chloride Cobalt(II) chloride Cobalt chloride is an inorganic compound of cobalt and chloride, with the formula CoCl2. It is usually supplied as the hexahydrate CoCl2·6H2O, which is one of the most commonly used cobalt compounds in the laboratory. The hexahydrate is deep purple in color, whereas the anhydrous form is sky blue... | CoCl2 | Deep blue | _chloride.jpg) |
| Cobalt(II) chloride hexahydrate | CoCl2 · 6H2O | Deep magenta | _chloride_hexahydrate.jpg) |
| Manganese(II) chloride Manganese(II) chloride Manganese chloride describes a series of compounds with the formula MnCl2x, where the value of x can be 0, 2, or 4. The tetrahydrate is the most common form of "manganese chloride". MnCl2·4H2O, but the anhydrous form and dihydrate MnCl2·2H2O are also known... tetrahydrate | MnCl2 · 4H2O | Pink | _chloride_tetrahydrate.jpg) |
| Copper(II) chloride Copper(II) chloride Copper chloride is the chemical compound with the formula CuCl2. This is a light brown solid, which slowly absorbs moisture to form a blue-green dihydrate. The copper chlorides are some of the most common copper compounds, after copper sulfate.... dihydrate | CuCl2 · 2H2O | Blue-green | _chloride_dihydrate.jpg) |
| Nickel(II) chloride Nickel(II) chloride Nickel chloride , is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. It is very rarely found in nature as mineral nickelbischofite. A dihydrate is also known. In general nickel chloride, in various forms, is the most important source of... hexahydrate | NiCl2 · 6H2O | Green |  |
| Lead(II) iodide Lead(II) iodide Lead iodide or plumbous iodide is a bright yellow solid at room temperature, that reversibly becomes brick red by heating. In its crystalline form it is used as a detector material for high energy photons including x-rays and gamma rays.... | PbI2 | Yellow |
Ions in Flame
Flame Tests on cations for Alkali, Alkali Earth Metals, and Hydrogen (see atomic spectroscopyAtomic spectroscopy
Atomic spectroscopy is the determination of elemental composition by its electromagnetic or mass spectrum. Atomic spectroscopy is closely related to other forms of spectroscopy. It can be divided by atomization source or by the type of spectroscopy used. In the latter case, the main division is...
) (see also Flame test
Flame test
A flame test is a procedure used in chemistry to detect the presence of certain metal ions, based on each element's characteristic emission spectrum. The color of flames in general also depends on temperature; see flame color....
)
Metals
| Name | Formula | Color |
|---|---|---|
| Potassium | K | Lilac/Purple |
| Sodium | Na | Yellow |
| Lithium | Li | Red |
| Caesium | Cs | Blue |
| Calcium | Ca | Red/Orange |
| Strontium | Sr | Red |
| Barium | Ba | Green/Yellow |
Gases
| Name | Formula | Color |
|---|---|---|
| Hydrogen | H2 | colorless |
Bead tests
A variety of colors, often similar to the colors found in a flame test, are produced in a bead test, which is a qualitative test for determining metals. A platinum loop is moistened and dipped in a fine powder of the substance in question and boraxBorax
Borax, also known as sodium borate, sodium tetraborate, or disodium tetraborate, is an important boron compound, a mineral, and a salt of boric acid. It is usually a white powder consisting of soft colorless crystals that dissolve easily in water.Borax has a wide variety of uses...
. The loop with the adhered powders is then heated in a flame until it fusses and the color of the resulting bead observed.
| Metal Metal A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light... |
Oxidising flame | Reducing flame |
|---|---|---|
| Aluminum | colorless (hot and cold), opaque | colorless, opaque |
| Antimony Antimony Antimony is a toxic chemical element with the symbol Sb and an atomic number of 51. A lustrous grey metalloid, it is found in nature mainly as the sulfide mineral stibnite... |
colorless, yellow or brown (hot) | gray and opaque |
| Barium Barium Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with... |
colorless | |
| Bismuth Bismuth Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead... |
colorless, yellow or brownish (hot) | gray and opaque |
| Cadmium Cadmium Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low... |
colorless | gray and opaque |
| Calcium Calcium Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust... |
colorless | |
| Cerium Cerium Cerium is a chemical element with the symbol Ce and atomic number 58. It is a soft, silvery, ductile metal which easily oxidizes in air. Cerium was named after the dwarf planet . Cerium is the most abundant of the rare earth elements, making up about 0.0046% of the Earth's crust by weight... |
red (hot) | colorless (hot and cold) |
| Chromium Chromium Chromium is a chemical element which has the symbol Cr and atomic number 24. It is the first element in Group 6. It is a steely-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odorless, tasteless, and malleable... |
Dark yellow (hot), green (cold) | green (hot and cold) |
| Cobalt Cobalt Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal.... |
blue (hot and cold) | blue (hot and cold) |
| Copper Copper Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish... |
green (hot), blue (cold) | red, opaque (cold), colorless (hot) |
| Iron Iron Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust... |
yellow or brownish red (hot and cold) | green (hot and cold) |
| Lead Lead Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed... |
colorless, yellow or brownish (hot) | gray and opaque |
| Magnesium Magnesium Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole... |
colorless | |
| Manganese Manganese Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals... |
|violet (hot and cold) | colorless (hot and cold) |
| Molybdenum Molybdenum Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores... |
colorless | yellow or brown (hot) |
| Nickel Nickel Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile... |
brown, red (cold) | gray and opaque (cold) |
| Silicon Silicon Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table... |
colorless (hot and cold), opaque | colorless, opaque |
| Silver Silver Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal... |
colorless | gray and opaque |
| Strontium Strontium Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and... |
colorless | |
| Tin Tin Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4... |
colorless (hot and cold), opaque | colorless, opaque |
| Titanium Titanium Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color.... |
colorless | yellow (hot), biolet (cold) |
| Tungsten Tungsten Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as... |
colorless | brown |
| Uranium Uranium Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons... |
Yellow or brownish (hot) | green |
| Vanadium Vanadium Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery gray, ductile and malleable transition metal. The formation of an oxide layer stabilizes the metal against oxidation. The element is found only in chemically combined form in nature... |
colorless | green |

