
Zinc-zinc oxide cycle
Encyclopedia
Thermochemical cycle
Thermochemical cycles combine solely heat sources with chemical reactions to split water into its hydrogen and oxygen components . The term cycle is used because aside of water, hydrogen and oxygen, the chemical compounds used in these processes are continuously recycled.If work is partially used...
based on zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
and zinc oxide
Zinc oxide
Zinc oxide is an inorganic compound with the formula ZnO. It is a white powder that is insoluble in water. The powder is widely used as an additive into numerous materials and products including plastics, ceramics, glass, cement, rubber , lubricants, paints, ointments, adhesives, sealants,...
for hydrogen production
Hydrogen production
Hydrogen production is the family of industrial methods for generating hydrogen. Currently the dominant technology for direct production is steam reforming from hydrocarbons. Many other methods are known including electrolysis and thermolysis...
with a typical efficiency around 40%.
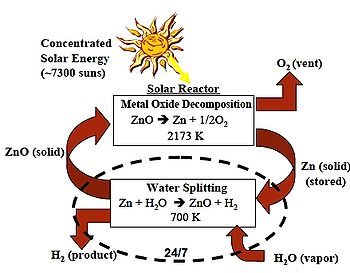
Process description
The thermochemical two-step water splittingWater splitting
Water splitting is the general term for a chemical reaction in which water is separated into oxygen and hydrogen. Efficient and economical water splitting would be a key technology component of a hydrogen economy. Various techniques for water splitting have been issued in water splitting patents in...
process uses redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
systems:
- DissociationDissociation (chemistry)Dissociation in chemistry and biochemistry is a general process in which ionic compounds separate or split into smaller particles, ions, or radicals, usually in a reversible manner...
: ZnOZinc oxideZinc oxide is an inorganic compound with the formula ZnO. It is a white powder that is insoluble in water. The powder is widely used as an additive into numerous materials and products including plastics, ceramics, glass, cement, rubber , lubricants, paints, ointments, adhesives, sealants,...
→ ZnZincZinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
+ 1/2 O2
- HydrolysisHydrolysisHydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
: ZnZincZinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
+ H2O → ZnOZinc oxideZinc oxide is an inorganic compound with the formula ZnO. It is a white powder that is insoluble in water. The powder is widely used as an additive into numerous materials and products including plastics, ceramics, glass, cement, rubber , lubricants, paints, ointments, adhesives, sealants,...
+ H2HydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
For the first endothermic
Endothermic
In thermodynamics, the word endothermic describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat. Its etymology stems from the prefix endo- and the Greek word thermasi,...
step concentrating solar power is used in which zinc oxide is thermally dissociated
Thermal decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes....
at 1900 °C (3,452 °F) into zinc and oxygen. In the second non-solar exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
step zinc reacts at 427 °C (800.6 °F) with water and produces hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and zinc oxide. The temperature level is realized by using a solar power tower
Solar power tower
The solar power tower is a type of solar furnace using a tower to receive the focused sunlight. It uses an array of flat, movable mirrors to focus the sun's rays upon a collector tower...
and a set of heliostat
Heliostat
A heliostat is a device that includes a mirror, usually a plane mirror, which turns so as to keep reflecting sunlight toward a predetermined target, compensating for the sun's apparent motions in the sky. The target may be a physical object, distant from the heliostat, or a direction in space...
s to collect the solar thermal energy
Solar thermal energy
Solar thermal energy is a technology for harnessing solar energy for thermal energy . Solar thermal collectors are classified by the United States Energy Information Administration as low-, medium-, or high-temperature collectors. Low-temperature collectors are flat plates generally used to heat...
.
See also
- Cerium(IV) oxide–cerium(III) oxide cycle
- Copper–chlorine cycle
- Hydrosol-2Hydrosol-2HYDROSOL is series of European Union funded projects for the promotion of renewable energy...
- Hybrid sulfur cycleHybrid sulfur cycleThe hybrid sulfur cycle is a two-step water-splitting process intended to be used for hydrogen production. Based on sulfur oxidation and reduction, it is classified as a hybrid thermochemical cycle because it uses an electrochemical reaction for one of the two steps...
- Iron oxide cycleIron oxide cycleThe iron oxide cycle is a two-step thermochemical cycle proposed for use for hydrogen production.-Process description:The thermochemical two-step water splitting process uses redox systems...
- Sulfur–iodine cycle

