Thiourea
Encyclopedia
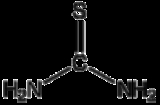
Thiourea is an organosulfur compound of with the formula
S
C
(N
H
2)2 . It is structurally similar to urea
, except that the oxygen
atom is replaced by a sulfur
atom, but the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis
. "Thioureas" refers to a broad class of compounds with the general structure (R1R2N)(R3R4N)C=S. Thioureas are related to thioamide
s, e.g. RC(S)NR2, where R is methyl, ethyl
, etc.
Thiourea occurs in two tautomer
ic forms. In aqueous solution, the thione shown on the left below predominates:
, but more commonly it is produced by the reaction of hydrogen sulfide
with calcium cyanamide
in the presence of carbon dioxide
.
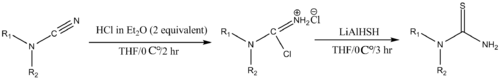
Many thiourea derivatives are useful. N,N-unsubstituted thioureas are generally prepared by allowing the corresponding cyanamide to react with LiAlHSH in the presence of 1 N HCl in anhydrous diethyl ether
. LiAlHSH can be prepared by reacting sulfur
with lithium aluminium hydride
.
s. The intermediate of the reaction is an unstable epidioxide which can only be identified at . Epidioxide is similar to epoxide
except with two oxygen atoms. This intermediate reduces to diol by thiourea.
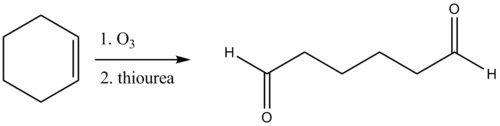
 Thiourea is also used in the reductive workup of ozonolysis
Thiourea is also used in the reductive workup of ozonolysis
to give carbonyl
compounds. Dimethyl sulfide
is also an effective reagent for this reaction, but it is highly volatile (b.p. ) and has an obnoxious odor whereas thiourea is odorless and conveniently non-volatile (reflecting its polarity).
In this example, ethane-1,2-dithiol is prepared from 1,2-dibromopropane
:
Thiourea is a precursor to sulfide to produce metal sulfides, e.g. mercury sulfide
, upon reaction with the metal salt in aqueous solution.
derivatives. Thus thioureas condense with β-dicarbonyl compounds. The amino group on the thiourea initially condenses with a carbonyl, followed by cyclization and tautomerization. Desulfurization delivers the pyrimidine.
Similarly, aminothiazoles can be synthesized by the reaction of alpha-halo ketone
s and thiourea.
The pharmaceuticals thiobarbituric acid
and sulfathiazole
is prepared using thiourea.
, and sulfamic acid
. A lixiviant
for gold and silver leaching can be created by selectively oxidizing thiourea, bypassing the steps of cyanide use and smelting.
resins, and vulcanization
accelerators. Thiourea is used as an auxiliary agent in diazo paper (light-sensitive photocopy paper) and almost all other types of copy paper.
, noxytiolin
and Burimamide
. Thioureas are catalysts in thiourea organocatalysis
.
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
S
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
(N
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
H
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
2)2 . It is structurally similar to urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
, except that the oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
atom is replaced by a sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
atom, but the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. "Thioureas" refers to a broad class of compounds with the general structure (R1R2N)(R3R4N)C=S. Thioureas are related to thioamide
Thioamide
Thioamide is a functional group with the general structure R-CS-NR'R, where R, R', and R are organic groups. They are analogous to amides but they exhibit greater multiple bond character along the C-N bond, resulting in a larger rotational barrier...
s, e.g. RC(S)NR2, where R is methyl, ethyl
Ethyl group
In chemistry, an ethyl group is an alkyl substituent derived from ethane . It has the formula -C2H5 and is very often abbreviated -Et.Ethylation is the formation of a compound by introduction of the ethyl functional group, C2H5....
, etc.
Structure and bonding
Thiourea is a planar molecule. The C=S bond distance is for thiourea (as well as many of its derivatives). The material has the unusual property of changing to ammonium thiocyanate upon heating above . Upon cooling, the ammonium salt converts back to thiourea.Thiourea occurs in two tautomer
Tautomer
Tautomers are isomers of organic compounds that readily interconvert by a chemical reaction called tautomerization. This reaction commonly results in the formal migration of a hydrogen atom or proton, accompanied by a switch of a single bond and adjacent double bond...
ic forms. In aqueous solution, the thione shown on the left below predominates:
Production
The global annual production of thiourea is around 10,000 tons. About 40% is produced in Germany, another 40% in China, and 20% in Japan. Thiourea is produced from ammonium thiocyanateAmmonium thiocyanate
Ammonium thiocyanate is an inorganic compound with the formula NH4SCN. It is the salt of the ammonium cation and the thiocyanate anion.-Uses:...
, but more commonly it is produced by the reaction of hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
with calcium cyanamide
Calcium cyanamide
Calcium cyanamide or CaCN2 is a calcium compound used as fertilizer, first synthesized in 1898 by Adolph Frank and Nikodem Caro . It is formed when calcium carbide reacts with nitrogen. It is commercially known as Nitrolime....
in the presence of carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
.
Many thiourea derivatives are useful. N,N-unsubstituted thioureas are generally prepared by allowing the corresponding cyanamide to react with LiAlHSH in the presence of 1 N HCl in anhydrous diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
. LiAlHSH can be prepared by reacting sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
with lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
.
Organic synthesis
Thiourea reduces peroxides to the corresponding diolDiol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
s. The intermediate of the reaction is an unstable epidioxide which can only be identified at . Epidioxide is similar to epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
except with two oxygen atoms. This intermediate reduces to diol by thiourea.

Ozonolysis
Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
to give carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compounds. Dimethyl sulfide
Dimethyl sulfide
Dimethyl sulfide or methylthiomethane is an organosulfur compound with the formula 2S. Dimethyl sulfide is a water-insoluble flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize,...
is also an effective reagent for this reaction, but it is highly volatile (b.p. ) and has an obnoxious odor whereas thiourea is odorless and conveniently non-volatile (reflecting its polarity).

Source of sulfide
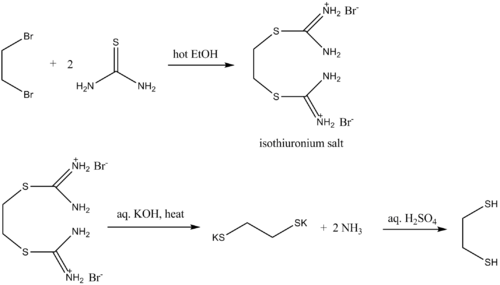
Thiourea is commonly employed as a source of sulfide, e.g. for converting alkyl halides to thiols. Such reactions proceed via the intermediacy of isothiuronium salts. The reaction capitalizes on the high nucleophilicity of the sulfur center and the hydrolytic instability of the isothiuronium salt:- CS(NH2)2 + RX → RSC(NH2)2+X-
- RSC(NH2)2+X- + 2 NaOH → RSNa + OC(NH2)2 + NaX
- RSNa + HCl → RSH + NaCl
In this example, ethane-1,2-dithiol is prepared from 1,2-dibromopropane
1,2-Dibromopropane
1,2-Dibromopropane, also known as propylene dibromide, is an organic compound with the formula CH3CHBrCH2Br. It is the simplest chiral hydrocarbon containing two bromine atoms:...
:
Thiourea is a precursor to sulfide to produce metal sulfides, e.g. mercury sulfide
Mercury sulfide
Mercury sulfide, mercuric sulfide, or mercury sulfide is a chemical compound composed of the chemical elements mercury and sulfur. It is represented by the chemical formula HgS...
, upon reaction with the metal salt in aqueous solution.
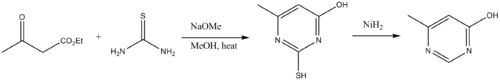
Precursor to heterocycles
Thioureas are used a building blocks to pyrimidinePyrimidine
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
derivatives. Thus thioureas condense with β-dicarbonyl compounds. The amino group on the thiourea initially condenses with a carbonyl, followed by cyclization and tautomerization. Desulfurization delivers the pyrimidine.
Similarly, aminothiazoles can be synthesized by the reaction of alpha-halo ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s and thiourea.
The pharmaceuticals thiobarbituric acid
Thiobarbituric acid
Thiobarbituric acid is an organic compound and a heterocycle. It is used as a reagent in assaying malondialdehyde .TBARS are higher in people who have headaches...
and sulfathiazole
Sulfathiazole
Sulfathiazole is an organosulfur compound that has been used as a short-acting sulfa drug. It once was a common oral and topical antimicrobial until less toxic alternatives were discovered. It is still occasionally used, sometimes in combination with sulfabenzamide and sulfacetamide, and in...
is prepared using thiourea.
Silver polishing
According to the label on the consumer product, the liquid silver cleaning product TarnX contains thiourea, a detergentDetergent
A detergent is a surfactant or a mixture of surfactants with "cleaning properties in dilute solutions." In common usage, "detergent" refers to alkylbenzenesulfonates, a family of compounds that are similar to soap but are less affected by hard water...
, and sulfamic acid
Sulfamic acid
Sulfamic acid, also known as amidosulfonic acid, amidosulfuric acid, aminosulfonic acid, and sulfamidic acid, is a molecular compound with the formula H3NSO3...
. A lixiviant
Lixiviant
Lixiviant is a liquid medium used in hydrometallurgy to selectively extract the desired metal from the ore or mineral. It assists in rapid and complete leaching. The metal can be recovered from it in a concentrated form after leaching. Lixiviant in a solution may be acidic or basic in nature. Most...
for gold and silver leaching can be created by selectively oxidizing thiourea, bypassing the steps of cyanide use and smelting.
Other uses
Other industrial uses of thiourea include production of flame retardantFlame retardant
Flame retardants are chemicals used in thermoplastics, thermosets, textiles and coatings that inhibit or resist the spread of fire. These can be separated into several different classes of chemicals:...
resins, and vulcanization
Vulcanization
Vulcanization or vulcanisation is a chemical process for converting rubber or related polymers into more durable materials via the addition of sulfur or other equivalent "curatives." These additives modify the polymer by forming crosslinks between individual polymer chains. Vulcanized material is...
accelerators. Thiourea is used as an auxiliary agent in diazo paper (light-sensitive photocopy paper) and almost all other types of copy paper.
Thioureas
Thioureas are compounds containing the thiourea motif. Examples are sulfathioureaSulfathiourea
Sulfathiourea is a sulfonamide antibacterial....
, noxytiolin
Noxytiolin
Noxytiolin is an anti-infective.*It is also used in certain medical procedures, mostly involving fusing a part of an animal with human flesh or bone to create stability....
and Burimamide
Burimamide
Burimamide is an antagonist at the H2 and H3 histamine receptors. It is largely inactive as an H2 antagonist at physiological pH, but its H3 affinity is 100x higher. It is a thiourea derivative....
. Thioureas are catalysts in thiourea organocatalysis
Thiourea organocatalysis
urea organocatalysis describes the utilization of properly designed derivatives of urea and - preferably - thiourea to accelerate and stereochemically alter organic transformations through predominantly double hydrogen-bonding interactions with the respective substrate...
.
Safety
The for thiourea is for rats (oral). A goitrogenic effect (enlargement of the thyroid gland) has been reported for chronic exposure, reflecting the ability of thiourea to interfere with iodide uptake.Further reading
- The Chemistry of double-bonded functional groups edited by S. Patai. pp 1355–1496. John Wiley & Sons. New York, NY, 1977. ISBN 0-471-92493-8.