
Tert-Butyl chloride
Encyclopedia
tert-Butyl chloride is a colorless, liquid organic compound
at room temperature
. It is sparingly soluble in water
, with a tendency to undergo spontaneous solvolysis
when dissolved
into it. The compound is flammable and volatile
, and its main use is as a starting molecule to carry out nucleophilic substitution reactions
, to produce different substances, ranging from alcohols to alkoxide
salts.
When tert-butyl chloride is dissolved in water, a polar
and protic solvent
, the bulky chloride
substituent
is carried away by it, and isolated from the aliphatic chain
, causing an heterolytic rupture of the compound, giving rise to a carbocation
which eventually becomes a tertiary alcohol after a water molecule reacts with it, releasing hydrochloric acid
as the final product. If a different, stronger nucleophilic agent is present at the moment of reaction, reaction product may not be an alcohol, but a tertiary carbon with the nucleophile
as a substituent.
with concentrated hydrochloric acid
, as shown below.
The overall reaction, therefore, is:
Because tert-butanol is a tertiary alcohol, the relative stability of the tert-butyl carbocation in the Step 2 allows the SN1 mechanism to be followed, whereas a primary alcohol would follow an SN2 mechanism.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
at room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
. It is sparingly soluble in water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, with a tendency to undergo spontaneous solvolysis
Solvolysis
Solvolysis is a special type of nucleophilic substitution or elimination where the nucleophile is a solvent molecule. For certain nucleophiles, there are specific terms for the type of solvolysis reaction...
when dissolved
Solvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
into it. The compound is flammable and volatile
Volatile organic compound
Volatile organic compounds are organic chemicals that have a high vapor pressure at ordinary, room-temperature conditions. Their high vapor pressure results from a low boiling point, which causes large numbers of molecules to evaporate or sublimate from the liquid or solid form of the compound and...
, and its main use is as a starting molecule to carry out nucleophilic substitution reactions
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
, to produce different substances, ranging from alcohols to alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
salts.
When tert-butyl chloride is dissolved in water, a polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
and protic solvent
Protic solvent
In chemistry a protic solvent is a solvent that has a hydrogen atom bound to an oxygen or a nitrogen . In general terms, any molecular solvent that contains dissociable H+ is called a protic solvent. The molecules of such solvents can donate an H+...
, the bulky chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
is carried away by it, and isolated from the aliphatic chain
Aliphatic compound
In organic chemistry, aliphatic compounds are acyclic or cyclic, non-aromatic carbon compounds.Thus, aliphatic compounds are opposite to aromatic compounds.- Structure :...
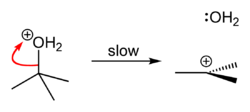
, causing an heterolytic rupture of the compound, giving rise to a carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
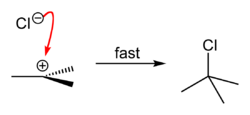
which eventually becomes a tertiary alcohol after a water molecule reacts with it, releasing hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
as the final product. If a different, stronger nucleophilic agent is present at the moment of reaction, reaction product may not be an alcohol, but a tertiary carbon with the nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
as a substituent.
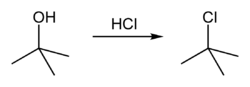
Synthesis
tert-Butyl chloride can be synthesized in the laboratory by the SN1 reaction of tert-ButanolTert-Butanol
tert-Butanol, or 2-methyl-2-propanol, is the simplest tertiary alcohol. It is one of the four isomers of butanol. tert-Butanol is a clear liquid with a camphor-like odor. It is very soluble in water and miscible with ethanol and diethyl ether...
with concentrated hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
, as shown below.
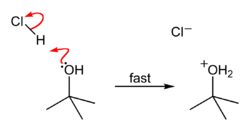 |
 |
 |
Leaving group In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate... (water). |
Carbocation A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,... . |
The overall reaction, therefore, is:

Because tert-butanol is a tertiary alcohol, the relative stability of the tert-butyl carbocation in the Step 2 allows the SN1 mechanism to be followed, whereas a primary alcohol would follow an SN2 mechanism.

