
Surface stress
Encyclopedia
Surface stress was first defined by Josiah Willard Gibbs
as the amount of reversible work per unit area needed to elastically stretch a pre-existing surface
. A similar term called “surface free energy”, which represents the excess free energy
per unit area needed to create a new surface, is easily confused with “surface stress”. Although surface stress and surface free energy of liquid-gas or liquid-liquid interface
are the same, they are very different in solid–gas or solid–solid interface, which will be discussed in details later. Since both terms represent a force
per unit length
, they have been referred to as “surface tension
”, which contributes further to the confusion in the literature.
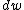 performed to create new area
performed to create new area 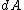 of surface, expressed as:
of surface, expressed as:
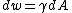
Gibbs was the first to define another surface quantity, different from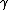 , that is associated with the reversible work per unit area needed to elastically stretch a pre-existing surface. Surface stress can be derived from surface free energy as followed :
, that is associated with the reversible work per unit area needed to elastically stretch a pre-existing surface. Surface stress can be derived from surface free energy as followed :
One can define a surface stress tensor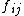 that relates the work associated with the variation in
that relates the work associated with the variation in 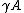 , the total excess free energy of the surface, owing to the strain
, the total excess free energy of the surface, owing to the strain 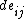 :
:

Now let's consider the two reversible paths showed in figure 0. The first path (clockwise), the solid object is cut into two same pieces. Then both pieces are elastically strained. The work associated with the first step (unstrained) is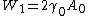 , where
, where 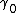 and
and 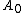 are the excess free energy and area of each of new surfaces. For the second step, work (
are the excess free energy and area of each of new surfaces. For the second step, work (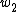 ), equals the work needed to elastically deform the total bulk volume and the four (two original and two newly formed) surfaces.
), equals the work needed to elastically deform the total bulk volume and the four (two original and two newly formed) surfaces.
In the second path (counter-clockwise), the subject is first elastically strained and then is cut in two pieces. The work for the first step here,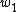 is equal to that needed to deform the bulk volume and the two surfaces. The difference
is equal to that needed to deform the bulk volume and the two surfaces. The difference 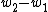 is equal to the excess work needed to elastically deform two surfaces of area
is equal to the excess work needed to elastically deform two surfaces of area 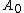 to area
to area  or:
or:
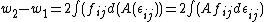
the work associated with the second step of the second path can be expressed as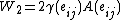 , so that:
, so that:
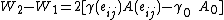
These two paths are completely reversible, or W2 – W1 = w2 – W1. It means:

Since d(γA) = γdA + Adγ , and dA = Aδijdeij. Then surface stress can be expressed as:
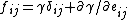
Where δij is the Kronecker delta and eij is elastic strain tensor.
Differently to the surface free energy γ, which is a scalar, surface stress fij is a second rank tensor. However, for a general surface, set of principle axes that are off-diagonal components are identically zero. Surface that possesses a threefold or higher rotation axis symmetry
, diagonal
components are equal. Therefore, surface stress can be rewritten as a scalar:

Now it can be easily explained why f and γ are equal in liquid-gas or liquid-liquid interfaces. Due to the chemical structure of liquid surface phase, the term ∂γ/∂e always equals to zero meaning that surface free energy won’t change even if the surface is being stretched. However, ∂γ/∂e is not zero in solid surface due do the fact that surface atomic structure of solid are modified in elastic deformation.
is very different from in the bulk
. Therefore, equilibrium
interatomic distance between surface atoms is different from bulk atoms. Since surface and bulk atoms are structurally coherent
, the interior of the solid can be considered as applying a stress on the surface.
For illustration, figure 1 shows a simple picture of bond charges near the surface of a 2-dimensional crystal
with charge (election) density around sphere atoms. Surface atoms only have two nearest neighbors compared with bulk atoms, which have four (for this example case). The loss of neighbors which results from the creation of a metal surface reduces the local electron density
around the atoms near the surface. Surface atoms then sit in a lower average electron density than bulk atoms. The response of these surface atoms would be to attempt to reduce their interatomic distance in order to increase surrounding charge density. Therefore, surface atoms would create a positive surface stress (tensile). In the other words, if the surface charge density is the same as in the bulk, surface stress would be zero.
Surface stress, which created by redistribution of electron density around surface atoms, can be both positive (tensile) or negative (compressive). If the surface is not clean meaning there are atoms sitting on a flat surface (adsorbates), charge density would then be modified leading to a different surface stress state compared with a perfect clean surface.
, atomistic potential calculations and molecular dynamics
simulations. Most of calculations are done at temperature
of 0 K. Following are tables of surface stress and surface free energy values of metals and semiconductors. Details of these calculations could be found in the attached references.
of a thin membrane of the material as it bends by gravitation through its own weight. This method turned out to be difficult since it requires a complete homogeneous
single crystal
surface. An alternative way to measure absolute surface stress is to measure the elastic extension of the length of the thin wire under an applied force. However, this method had many limitations itself and wasn’t used popularly. While determination of the absolute surface stress is still a challenge, the experimental technique to measure changes in the surface stress due to external interaction is well established using “cantilever bending method”. The principle of the measurement is shown in figure 2. In this case, stress of one surface is changed upon deposition of material which results the bending of the cantilever. The surface wants to expand creating a compressive stress. The radius of curvature R is measured as the change of the gap of a capacitor by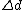 . Figure 2b shows the two electrodes of the capacitor
. Figure 2b shows the two electrodes of the capacitor
formed by the sample and a capacitor electrode c. The capacitor electrode is surrounded by a guard electrode in order to minimize the effects of stray capacitances. The sample b is clamped on one end in the sample holder a. The bending can also be measured with high sensitivity by deflection
of the beam of a laser
using a position sensitive detector. To use this method, it requires that the sample is thin enough. Some experiment measurement values are listed in table 5.
of the reconstruction is still not very clear.
Most of metallic surface reconstruction exhibit in two genetic forms. On the original (100) surface, it would form a hexagonal overlayer which results in a considerably higher density of surface atoms by 20–25%. On the original (111) surface, since it already in closed-pack structure, the higher density
is due to a contraction while the local coordination of the surface atoms remains a hexagonal one. Another way to explain the surface reconstruction phenomenon is called “soft phonon type of reconstruction”. The driving force for a change in the surface concentration associated with a contraction of the surface is proportional to the difference between surface stress and surface free energy. It corresponds to the amount of energy gained by structure transformation to over the surface stress. For semiconductor surface, forming dimer is the way for it to response to the tensile stress. Figure 3 shows an example of Si(100) surface reconstruction that create tensile stress.
Sij & exp(-crij)
where rij is distance between two adsorbate i and j
One can easily relate the mean distance between two adsorbates with square root of the coverage:
Sij & exp(-c/√θ)
Then the stress induced by absorbates can be derived as:
∆τ=a.θ+b.exp(-c/√θ) (8)
where a, b, and c are fitting parameters. Figure 4 shows very good fits for all systems with the equation 8.
However, later research shows that direct repulsive interaction between absorbate atoms (as well as dipolar interactions) contribute very little to the induced surface stress. The stress can become large only if the distance between the adsorbed atoms becomes small so that φij (repulsive pairwise interaction potential) becomes large. It rarely happens without very high gas pressure since adsorbated state become unstable with respect to desorption.
Josiah Willard Gibbs
Josiah Willard Gibbs was an American theoretical physicist, chemist, and mathematician. He devised much of the theoretical foundation for chemical thermodynamics as well as physical chemistry. As a mathematician, he invented vector analysis . Yale University awarded Gibbs the first American Ph.D...
as the amount of reversible work per unit area needed to elastically stretch a pre-existing surface
Surface
In mathematics, specifically in topology, a surface is a two-dimensional topological manifold. The most familiar examples are those that arise as the boundaries of solid objects in ordinary three-dimensional Euclidean space R3 — for example, the surface of a ball...
. A similar term called “surface free energy”, which represents the excess free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
per unit area needed to create a new surface, is easily confused with “surface stress”. Although surface stress and surface free energy of liquid-gas or liquid-liquid interface
Interface (chemistry)
An interface is a surface forming a common boundary among two different phases, such as an insoluble solid and a liquid, two immiscible liquids or a liquid and an insoluble gas. The importance of the interface depends on which type of system is being treated: the bigger the quotient area/volume,...
are the same, they are very different in solid–gas or solid–solid interface, which will be discussed in details later. Since both terms represent a force
Force
In physics, a force is any influence that causes an object to undergo a change in speed, a change in direction, or a change in shape. In other words, a force is that which can cause an object with mass to change its velocity , i.e., to accelerate, or which can cause a flexible object to deform...
per unit length
Length
In geometric measurements, length most commonly refers to the longest dimension of an object.In certain contexts, the term "length" is reserved for a certain dimension of an object along which the length is measured. For example it is possible to cut a length of a wire which is shorter than wire...
, they have been referred to as “surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
”, which contributes further to the confusion in the literature.
Thermodynamics of surface stress
Definition of surface free energy is seemly the amount of reversible work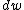 performed to create new area
performed to create new area 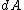 of surface, expressed as:
of surface, expressed as: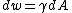
Gibbs was the first to define another surface quantity, different from
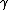 , that is associated with the reversible work per unit area needed to elastically stretch a pre-existing surface. Surface stress can be derived from surface free energy as followed :
, that is associated with the reversible work per unit area needed to elastically stretch a pre-existing surface. Surface stress can be derived from surface free energy as followed :One can define a surface stress tensor
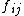 that relates the work associated with the variation in
that relates the work associated with the variation in 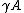 , the total excess free energy of the surface, owing to the strain
, the total excess free energy of the surface, owing to the strain 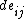 :
:
Now let's consider the two reversible paths showed in figure 0. The first path (clockwise), the solid object is cut into two same pieces. Then both pieces are elastically strained. The work associated with the first step (unstrained) is
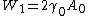 , where
, where 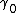 and
and 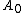 are the excess free energy and area of each of new surfaces. For the second step, work (
are the excess free energy and area of each of new surfaces. For the second step, work (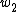 ), equals the work needed to elastically deform the total bulk volume and the four (two original and two newly formed) surfaces.
), equals the work needed to elastically deform the total bulk volume and the four (two original and two newly formed) surfaces.In the second path (counter-clockwise), the subject is first elastically strained and then is cut in two pieces. The work for the first step here,
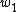 is equal to that needed to deform the bulk volume and the two surfaces. The difference
is equal to that needed to deform the bulk volume and the two surfaces. The difference 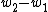 is equal to the excess work needed to elastically deform two surfaces of area
is equal to the excess work needed to elastically deform two surfaces of area 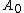 to area
to area  or:
or: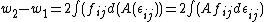
the work associated with the second step of the second path can be expressed as
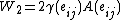 , so that:
, so that: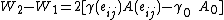
These two paths are completely reversible, or W2 – W1 = w2 – W1. It means:

Since d(γA) = γdA + Adγ , and dA = Aδijdeij. Then surface stress can be expressed as:
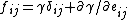
Where δij is the Kronecker delta and eij is elastic strain tensor.
Differently to the surface free energy γ, which is a scalar, surface stress fij is a second rank tensor. However, for a general surface, set of principle axes that are off-diagonal components are identically zero. Surface that possesses a threefold or higher rotation axis symmetry
Symmetry
Symmetry generally conveys two primary meanings. The first is an imprecise sense of harmonious or aesthetically pleasing proportionality and balance; such that it reflects beauty or perfection...
, diagonal
Diagonal
A diagonal is a line joining two nonconsecutive vertices of a polygon or polyhedron. Informally, any sloping line is called diagonal. The word "diagonal" derives from the Greek διαγώνιος , from dia- and gonia ; it was used by both Strabo and Euclid to refer to a line connecting two vertices of a...
components are equal. Therefore, surface stress can be rewritten as a scalar:

Now it can be easily explained why f and γ are equal in liquid-gas or liquid-liquid interfaces. Due to the chemical structure of liquid surface phase, the term ∂γ/∂e always equals to zero meaning that surface free energy won’t change even if the surface is being stretched. However, ∂γ/∂e is not zero in solid surface due do the fact that surface atomic structure of solid are modified in elastic deformation.
Physical origins of surface stress
Origin of surface stress could be understood by nature of chemical bonding of atoms at the surface. In metallic materials, atomic chemical bonding structure at the surfaceSurface
In mathematics, specifically in topology, a surface is a two-dimensional topological manifold. The most familiar examples are those that arise as the boundaries of solid objects in ordinary three-dimensional Euclidean space R3 — for example, the surface of a ball...
is very different from in the bulk
Bulk
-Industry:* Bulk cargo* Bulk liquids* Bulk mail* Bulk material handling* Bulk pack, packaged bulk materials/products* Bulk purchasing- Physics :*Bulk density*Bulk modulus...
. Therefore, equilibrium
Equilibrium
Equilibrium is the condition of a system in which competing influences are balanced. The word may refer to:-Biology:* Equilibrioception, the sense of a balance present in human beings and other animals...
interatomic distance between surface atoms is different from bulk atoms. Since surface and bulk atoms are structurally coherent
Coherence (physics)
In physics, coherence is a property of waves that enables stationary interference. More generally, coherence describes all properties of the correlation between physical quantities of a wave....
, the interior of the solid can be considered as applying a stress on the surface.
For illustration, figure 1 shows a simple picture of bond charges near the surface of a 2-dimensional crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
with charge (election) density around sphere atoms. Surface atoms only have two nearest neighbors compared with bulk atoms, which have four (for this example case). The loss of neighbors which results from the creation of a metal surface reduces the local electron density
Electron density
Electron density is the measure of the probability of an electron being present at a specific location.In molecules, regions of electron density are usually found around the atom, and its bonds...
around the atoms near the surface. Surface atoms then sit in a lower average electron density than bulk atoms. The response of these surface atoms would be to attempt to reduce their interatomic distance in order to increase surrounding charge density. Therefore, surface atoms would create a positive surface stress (tensile). In the other words, if the surface charge density is the same as in the bulk, surface stress would be zero.
Surface stress, which created by redistribution of electron density around surface atoms, can be both positive (tensile) or negative (compressive). If the surface is not clean meaning there are atoms sitting on a flat surface (adsorbates), charge density would then be modified leading to a different surface stress state compared with a perfect clean surface.
Theoretical calculations
Surface stresses normally calculated by calculating the surface free energy and its derivative with respect to elastic strain. Different methods have been used such as first principlesFirst principles
In philosophy, a first principle is a basic, foundational proposition or assumption that cannot be deduced from any other proposition or assumption. In mathematics, first principles are referred to as axioms or postulates...
, atomistic potential calculations and molecular dynamics
Molecular dynamics
Molecular dynamics is a computer simulation of physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a period of time, giving a view of the motion of the atoms...
simulations. Most of calculations are done at temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
of 0 K. Following are tables of surface stress and surface free energy values of metals and semiconductors. Details of these calculations could be found in the attached references.
Experimental measurements
In the early time, several experimental techniques to measure surface stress of materials had been proposed. One was determining surface stress by measuring curvatureCurvature
In mathematics, curvature refers to any of a number of loosely related concepts in different areas of geometry. Intuitively, curvature is the amount by which a geometric object deviates from being flat, or straight in the case of a line, but this is defined in different ways depending on the context...
of a thin membrane of the material as it bends by gravitation through its own weight. This method turned out to be difficult since it requires a complete homogeneous
Homogeneous (chemistry)
A substance that is uniform in composition is a definition of homogeneous. This is in contrast to a substance that is heterogeneous.The definition of homogeneous strongly depends on the context used. In Chemistry, a homogeneous suspension of material means that when dividing the volume in half, the...
single crystal
Single crystal
A single crystal or monocrystalline solid is a material in which the crystal lattice of the entire sample is continuous and unbroken to the edges of the sample, with no grain boundaries...
surface. An alternative way to measure absolute surface stress is to measure the elastic extension of the length of the thin wire under an applied force. However, this method had many limitations itself and wasn’t used popularly. While determination of the absolute surface stress is still a challenge, the experimental technique to measure changes in the surface stress due to external interaction is well established using “cantilever bending method”. The principle of the measurement is shown in figure 2. In this case, stress of one surface is changed upon deposition of material which results the bending of the cantilever. The surface wants to expand creating a compressive stress. The radius of curvature R is measured as the change of the gap of a capacitor by
 . Figure 2b shows the two electrodes of the capacitor
. Figure 2b shows the two electrodes of the capacitorCapacitor
A capacitor is a passive two-terminal electrical component used to store energy in an electric field. The forms of practical capacitors vary widely, but all contain at least two electrical conductors separated by a dielectric ; for example, one common construction consists of metal foils separated...
formed by the sample and a capacitor electrode c. The capacitor electrode is surrounded by a guard electrode in order to minimize the effects of stray capacitances. The sample b is clamped on one end in the sample holder a. The bending can also be measured with high sensitivity by deflection
Deflection (physics)
In physics deflection is the event where an object collides and bounces against a plane surface.In such collisions involving a sphere and a plane, the collision angle formed with the surface normal must equal the bounce angle , \alpha = \beta.Magnetic deflection refers to Lorentz forces acting...
of the beam of a laser
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
using a position sensitive detector. To use this method, it requires that the sample is thin enough. Some experiment measurement values are listed in table 5.
Surface structural reconstruction
Structural reconstruction at the surfaces has been studied extensively by both theoretical and experimental methods. However, a question about surface stress is high enough to be a main driving forceForce
In physics, a force is any influence that causes an object to undergo a change in speed, a change in direction, or a change in shape. In other words, a force is that which can cause an object with mass to change its velocity , i.e., to accelerate, or which can cause a flexible object to deform...
of the reconstruction is still not very clear.
Most of metallic surface reconstruction exhibit in two genetic forms. On the original (100) surface, it would form a hexagonal overlayer which results in a considerably higher density of surface atoms by 20–25%. On the original (111) surface, since it already in closed-pack structure, the higher density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
is due to a contraction while the local coordination of the surface atoms remains a hexagonal one. Another way to explain the surface reconstruction phenomenon is called “soft phonon type of reconstruction”. The driving force for a change in the surface concentration associated with a contraction of the surface is proportional to the difference between surface stress and surface free energy. It corresponds to the amount of energy gained by structure transformation to over the surface stress. For semiconductor surface, forming dimer is the way for it to response to the tensile stress. Figure 3 shows an example of Si(100) surface reconstruction that create tensile stress.
Adsorbate-induced changes in the surface stress
As mentioned above, surface stress is caused by charge density redistribution of surface atoms due to lacking of nearest neighbor atoms. In case of introduction of adsorbates (atoms that land on surface), charge density would be then modified around these adsorbates, resulting different surface stress state. There are many types of reaction between adsorbates and the surface that cause different stress behavior. Here, two most common behaviors are shown:Coverage dependence of the adsorbate-induced surface stress
Coverage effect of surface stress without surface reconstruction usually result a compressive stress (assuming clean clean surface as reference or zero stress). Induced surface stress of number of different coverages on Ni(100) and Pt(111) surface is shown in figure 4. In all cases, it shows an initially linear increase of the induced stress with coverage, followed by an increase larger than linear at higher coverages. The non-linear increase is first thought to be due to the repulsive interaction between adsorbates. The repulsive interaction should be proportional to the overlap integrals summed of non-bonding orbitals with exponential relationship:Sij & exp(-crij)
where rij is distance between two adsorbate i and j
One can easily relate the mean distance between two adsorbates with square root of the coverage:
Sij & exp(-c/√θ)
Then the stress induced by absorbates can be derived as:
∆τ=a.θ+b.exp(-c/√θ) (8)
where a, b, and c are fitting parameters. Figure 4 shows very good fits for all systems with the equation 8.
However, later research shows that direct repulsive interaction between absorbate atoms (as well as dipolar interactions) contribute very little to the induced surface stress. The stress can become large only if the distance between the adsorbed atoms becomes small so that φij (repulsive pairwise interaction potential) becomes large. It rarely happens without very high gas pressure since adsorbated state become unstable with respect to desorption.
Adsorbate-induced stress and restructuring of surfaces
It shows that the tensile stress on clean surfaces can be so strong that the surface reconstructs to form an overlayer of higher charge density. In the presence of adsorbates, stress induced by could also be high enough for such reconstruction. The mechanism of the reconstruction of the two processes would be similar. The reconstruction due to adsorbates is easily recognized by deviation from stress-induced vs. coverage relationship. One example is shown in figure 5 and 6. It shows clearly the difference between stress-induced behavior of silicon compared with oxygen or carbon absorbate on Ni(100) surface. S/Ni(100) system reaches very high stress at the coverage of ~0.3. This stress then causes a reconstruction (figure 5) to increase the charge density of surface atoms in order to reduce the developed stress.See also
- Gibbs free energyGibbs free energyIn thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
- Surface tensionSurface tensionSurface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
- Surface free energy
- First principlesFirst principlesIn philosophy, a first principle is a basic, foundational proposition or assumption that cannot be deduced from any other proposition or assumption. In mathematics, first principles are referred to as axioms or postulates...
calculation - Atomistic potential calculation
- Molecular dynamicsMolecular dynamicsMolecular dynamics is a computer simulation of physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a period of time, giving a view of the motion of the atoms...
simulations - Cantilever bending method
- Surface reconstruction
- NanocrystallineNanocrystallineA nanocrystalline material is a polycrystalline material with a crystallite size of only a few nanometers. These materials fill the gap between amorphous materials without any longe range order and crystalline materials with a clear three dimensional long range order.X-ray diffraction is commonly...
materials - NanowireNanowireA nanowire is a nanostructure, with the diameter of the order of a nanometer . Alternatively, nanowires can be defined as structures that have a thickness or diameter constrained to tens of nanometers or less and an unconstrained length. At these scales, quantum mechanical effects are important —...
materials - NanoporousNanoporousNanoporous materials consist of a regular organic or inorganic framework supporting a regular, porous structure. Pores are by definition roughly in the nanometre range, that is between 1x10−7 and 0.2x10−9 m.Subdivisions:...
materials

