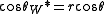Superhydrophobe
Encyclopedia

Nelumbo
Nelumbo is a genus of aquatic plants with large, showy flowers resembling water lilies, commonly known as lotus. The generic name is derived from the Sinhalese word Nelum. There are only two known living species in the genus. The sacred lotus is native to Asia, and is the better known of the two...
plant have surfaces that are highly hydrophobic, i.e., extremely difficult to wet. The contact angle
Contact angle
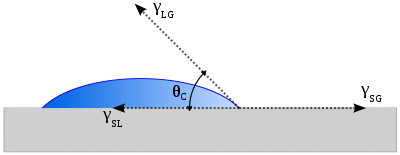
The contact angle is the angle at which a liquid/vapor interface meets a solid surface. The contact angle is specific for any given system and is determined by the interactions across the three interfaces. Most often the concept is illustrated with a small liquid droplet resting on a flat...
s of a water droplet exceeds 150° and the roll-off angle is less than 10°. This is referred to as the Lotus effect
Lotus effect
The lotus effect refers to the very high water repellence exhibited by the leaves of the lotus flower ....
.
Theory
In 1805, Thomas YoungThomas Young (scientist)
Thomas Young was an English polymath. He is famous for having partly deciphered Egyptian hieroglyphics before Jean-François Champollion eventually expanded on his work...
defined the contact angle θ by analyzing the forces acting on a fluid droplet resting on a solid surface surrounded by a gas.
-
- where
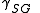 = Interfacial tension between the solid and gas
= Interfacial tension between the solid and gas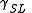 = Interfacial tension between the solid and liquid
= Interfacial tension between the solid and liquid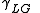 = Interfacial tension between the liquid and gas
= Interfacial tension between the liquid and gas
θ can be measured using a contact angle goniometer
Goniometer
A goniometer is an instrument that either measures an angle or allows an object to be rotated to a precise angular position. The term goniometry is derived from two Greek words, gōnia, meaning angle, and metron, meaning measure....
.
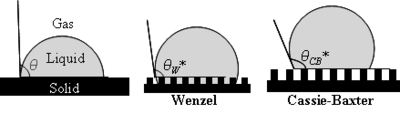
Wenzel determined that when the liquid is in intimate contact with a microstructured surface, θ will change to
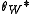
where r is the ratio of the actual area to the projected area. Wenzel's equation shows that microstructuring a surface amplifies the natural tendency of the surface. A hydrophobic surface (one that has an original contact angle greater than 90°) becomes more hydrophobic when microstructured – its new contact angle becomes greater than the original. However, a hydrophilic surface (one that has an original contact angle less than 90°) becomes more hydrophilic when microstructured – its new contact angle becomes less than the original.
Cassie and Baxter found that if the liquid is suspended on the tops of microstructures, θ will change to
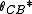
-
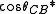 = φ(cos θ + 1) – 1
= φ(cos θ + 1) – 1
where φ is the area fraction of the solid that touches the liquid. Liquid in the Cassie-Baxter state is more mobile than in the Wenzel state.
It can be predicted whether the Wenzel or Cassie-Baxter state should exist by calculating the new contact angle with both equations. By a minimization of free energy argument, the relation that predicted the smaller new contact angle is the state most likely to exist. Stated mathematically, for the Cassie-Baxter state to exist, the following inequality must be true.
-
- cos θ < (φ-1)/(r - φ)
A recent alternative criteria for the Cassie-Baxter state asserts that the Cassie-Baxter state exists when the following 2 criteria are met: 1) Contact line forces overcome body forces of unsupported droplet weight and 2) The microstructures are tall enough to prevent the liquid that bridges microstructures from touching the base of the microstructures.
Contact angle is a measure of static hydrophobicity, and contact angle hysteresis and slide angle are dynamic measures. Contact angle hysteresis is a phenomenon that characterizes surface heterogeneity. When a pipette injects a liquid onto a solid, the liquid will form some contact angle. As the pipette injects more liquid, the droplet will increase in volume, the contact angle will increase, but its three phase boundary will remain stationary until it suddenly advances outward. The contact angle the droplet had immediately before advancing outward is termed the advancing contact angle. The receding contact angle is now measured by pumping the liquid back out of the droplet. The droplet will decrease in volume, the contact angle will decrease, but its three phase boundary will remain stationary until it suddenly recedes inward. The contact angle the droplet had immediately before receding inward is termed the receding contact angle. The difference between advancing and receding contact angles is termed contact angle hysteresis and can be used to characterize surface heterogeneity, roughness, and mobility. Surfaces that are not homogeneous will have domains which impede motion of the contact line. The slide angle is another dynamic measure of hydrophobicity and is measured by depositing a droplet on a surface and tilting the surface until the droplet begins to slide. Liquids in the Cassie-Baxter state generally exhibit lower slide angles and contact angle hysteresis than those in the Wenzel state.
A simple model can be used to predict the effectiveness of a manmade micro- or nano-fabricated surface for its conditional state (wenzel or cassie-baxter), contact angle and contact angle hysteresis. The main factor of this model is the contact line density, Λ, which is the total perimeter of asperities over a given unit area.
The critical contact line density Λc is a function of body and surface forces, as well as the projected area of the droplet.
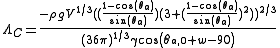
where
- ρ = density of the liquid droplet
- g = acceleration due to gravity
- V = volume of the liquid droplet
- θa = advancing appearant contact angle
- θa,0 = advancing contact angle of a smooth substrate
- γ = surface tension of the liquid
- w = tower wall angle
If Λ > Λc, drops are suspended in the cassie-baxter state. Otherwise, the droplet will collapse into the wenzel state.
To calculate updated advancing and receding contact angles in the cassie-baxter state, the following equations can be used.
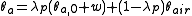
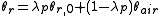
with also the wenzel state:
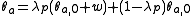
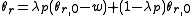
where
- λp = linear fraction of contact line on the asperities
- θr,0 = advancing contact angle of a smooth substrate
- θair = contact angle between liquid and air (typically assumed to be 180°)
Recent research
The self-cleaning property of superhydrophobic micro-nanostructuredNanotechnology
Nanotechnology is the study of manipulating matter on an atomic and molecular scale. Generally, nanotechnology deals with developing materials, devices, or other structures possessing at least one dimension sized from 1 to 100 nanometres...
surfaces was reported in 1977, and perfluoroalkyl and perfluoropolyether superhydrophobic materials were developed in 1986 for handling chemical and biological fluids. Other biotechnical applications have emerged since the 1990s.
Many very hydrophobic materials found in nature rely on Cassie's law
Cassie's law
Cassie's law describes the effective contact angle θc for a liquid on a composite surface . The law explains how simply roughing up a surface increases the apparent surface angle...
and are biphasic
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
on the submicrometer level with one component air. The Lotus effect
Lotus effect
The lotus effect refers to the very high water repellence exhibited by the leaves of the lotus flower ....
is based on this principle. Inspired by it, a lot of functional superhydrophobic surfaces were prepared.
Research in superhydrophobicity recently accelerated with a letter that reported man-made superhydrophobic samples produced by allowing alkylketene dimer (AKD) to solidify into a nanostructured fractal surface. Many papers have since presented fabrication methods for producing superhydrophobic surfaces including particle deposition, sol-gel techniques, plasma treatments, vapor deposition, and casting techniques. Current opportunity for research impact lies mainly in fundamental research and practical manufacturing. Debates have recently emerged concerning the applicability of the Wenzel and Cassie-Baxter models. In an experiment designed to challenge the surface energy perspective of the Wenzel and Cassie-Baxter model and promote a contact line perspective, water drops were placed on a smooth hydrophobic spot in a rough hydrophobic field, a rough hydrophobic spot in a smooth hydrophobic field, and a hydrophilic spot in a hydrophobic field. Experiments showed that the surface chemistry and geometry at the contact line affected the contact angle and contact angle hysteresis, but the surface area inside the contact line had no effect. An argument that increased jaggedness in the contact line enhances droplet mobility has also been proposed.
There have been a few efforts in fabricating a surface with tunable wettability. For the purpose of spontaneous droplet mobility, a surface can be fabricated with varying tower widths and spacings to gradually increase the free energy of the surface The trend shows that as tower width increases, the free energy barrier becomes larger and the contact angle drops, lowering the hydrophobicity of the material. In addition, increasing tower spacing will increase the contact angle, but also increase the free energy barrier. Droplets naturally move towards areas of weak hydrophobicity, so to make a droplet spontaneously move from one spot to the next, the ideal surface would consist of small width towers with large spacing to large width towers with small spacing. One caveat to this spontaneous motion is the resistance of stationary droplets to move. Initial droplet motion requires an external stimulus, from something as large as a vibration of the surface or as small as a simple syringe “push” as it is released from the needle.
An example of readily tunable wettability is found with special developed fabrics. By stretching a dip-coated commercial fabric, contact angles were typically allowed to increase. This is largely caused by an increase in tower spacing. However, this trend does not continue towards greater hydrophobicity with higher strain. Eventually, the cassie-baxter state reaches an instability and transitions to the wenzel state, soaking the fabric.
An example of a biomimetic
Bionics
Bionics is the application of biological methods and systems found in nature to the study and design of engineering systems and modern technology.The word bionic was coined by Jack E...
superhydrophobic material in nanotechnology
Nanotechnology
Nanotechnology is the study of manipulating matter on an atomic and molecular scale. Generally, nanotechnology deals with developing materials, devices, or other structures possessing at least one dimension sized from 1 to 100 nanometres...
is nanopin film
Nanopin film
Nanopin film is an experimental material in nanotechnology developed in 2005 with unusual superhydrophobic properties . A droplet of water makes contact with the surface of this film and forms an almost perfect sphere with a contact angle of 178°. The film is able to do this because it is covered...
. In one study a vanadium pentoxide surface is presented that can switch reversibly between superhydrophobicity and superhydrophilicity under the influence of UV radiation. According to the study any surface can be modified to this effect by application of a suspension
Suspension (chemistry)
In chemistry, a suspension is a heterogeneous fluid containing solid particles that are sufficiently large for sedimentation. Usually they must be larger than 1 micrometer. The internal phase is dispersed throughout the external phase through mechanical agitation, with the use of certain...
of rose-like V2O5 particles for instance with an inkjet printer
Inkjet printer
An inkjet printer is a type of computer printer that creates a digital image by propelling droplets of ink onto paper. Inkjet printers are the most commonly used type of printer and range from small inexpensive consumer models to very large professional machines that can cost up to thousands of...
. Once again hydrophobicity is induced by interlaminar air pockets (separated by 2.1 nm distances). The UV effect is also explained. UV light creates electron-hole pairs, with the holes reacting with lattice oxygen creating surface oxygen vacancies while the electrons reduce V5+ to V3+. The oxygen vacancies are met by water and this water absorbency by the vanadium surface makes it hydrophilic. By extended storage in the dark, water is replaced by oxygen and hydrophilicity is once again lost.
Another example of a biomimetic surface includes micro-flowers on common polymer polycarbonates. The micro/nano binary structures (MNBS) imitate the typical micro/nanostructure of a lotus leaf. These micro-flowers offer nanoscale features which enchance the surface's hydrophobicity, without the use of low surface energy coatings. Creation of the superhydrophobic surface through vapor-induced phase separation at varying surrounding relative humidities caused a likewise change to the contact angle of the surface. Surfaces prepared offer contact angles higher than 160° with typical sliding angles around 10°.
Low surface energy coatings can also provide a superhydrophobic surface. A self-assembled monolayer
Self-assembled monolayer
A self assembled monolayer is an organized layer of amphiphilic molecules in which one end of the molecule, the “head group” shows a specific, reversible affinity for a substrate...
(SAM) coating can provide such surfaces. To maintain a hydrophobic surface, the head groups bind closely to the surface, while the hydrophobic miscelles stretch far away from the surface. By varying the amount of SAM you coat on a substrate, one could vary the degree of hydrophobicity. Particular superhydrophobic SAMs have a hydrophobic head group binding to the substrate. In one such work, 1-dodecanethiol (DT; CH3(CH2)11SH) is assembled on a Pt/ZnO/SiO2 composite substrate, producing contact angles of 170.3°. The monolayers could also be removed with a UV source, decreasing the hydrophobicity.
Potential applications
Active recent research on superhydrophobic materials might eventually lead to industrial applications. Some attempts at fabricating a superhydrophobic surface include mimicking a lotus leaf surface, namely the two-tiered characteristic. This requires micro-scale surfaces with typically nanoscale features on top of them. For example, a simple routine of coating cotton fabric with silica or titaniaTitanium dioxide
Titanium dioxide, also known as titanium oxide or titania, is the naturally occurring oxide of titanium, chemical formula . When used as a pigment, it is called titanium white, Pigment White 6, or CI 77891. Generally it comes in two different forms, rutile and anatase. It has a wide range of...
particles by sol-gel technique has been reported, which protects the fabric from UV light and makes it superhydrophobic. Similarly, silica nanoparticles can be deposited on top of already hydrophobic carbon fabric. The carbon fabric by itself is identified as inherently hydrophobic, but not distinguished as superhydrophobic since its contact angle isn't higher than 150°. With the adhesion of silica nanoparticles, contact angles as high as 162° are achieved. Using silica nano-particles is also of interest to develop transparent hydrophobic materials for car windshields and self-cleaning windows. By coating an already transparent surface with nano-silica with about 1% wt., droplet contact angles can raise up to 168° with a 12° sliding angle.
In addition, an efficient routine has been reported for making polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
superhydrophobic and thus self-cleaning—99% of dirt adsorbed on such surface is easily washed away. Patterned superhydrophobic surfaces also have the promises for the lab-on-a-chip, microfluidic devices and can drastically improve the surface based bioanalysis.
In the textile industry, superhydrophobicity refers to static roll-off angles of water of 20° or less. An example of superhydrophobic effect in live application is the team Alinghi in America's Cup using specially treated sailing jackets. The treatment is built up by micrometre size particles in combination with traditional fluorine chemistry.
A recent application of hydrophobic structures and materials is in the development of micro fuel cell chips. Reactions within the fuel cell produce waste gas CO2 which can be vented out through these hydrophobic membranes. The membrane consists of many microcavities which allow the gas to escape, while its hydrophobicity characteristic prevents the liquid fuel from leaking through. More fuel flows in to replace the volume previously kept by the waste gas, and the reaction is allowed to continue.