
Contact angle
Encyclopedia
The contact angle is the angle at which a liquid
/vapor
interface meets a solid
surface. The contact angle is specific for any given system and is determined by the interactions across the three interfaces. Most often the concept is illustrated with a small liquid droplet resting on a flat horizontal solid surface. The shape of the droplet is determined by the Young-Laplace equation, with the contact angle playing the role of a boundary condition. Contact angle is measured using a contact angle goniometer
. The contact angle is not limited to a liquid/vapour interface; it is equally applicable to the interface of two liquids.
If the solid surface is hydrophobic, the contact angle will be larger than 90°. On highly hydrophobic surfaces the surfaces have water contact angles as high as ~120° on low energy materials e.g. fluorinated surfaces. However, some materials with highly rough surfaces may have a water contact angle greater than 150°. These are called superhydrophobic surfaces. Sometimes the contact angle is measured through the gas instead of through the liquid, which reverses 0 and 180 in the above explanation.
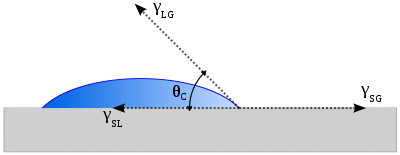 The theoretical description of contact arises from the consideration of a thermodynamic
The theoretical description of contact arises from the consideration of a thermodynamic
equilibrium
between the three phases
: the liquid
phase of the droplet (L), the solid
phase of the substrate (S), and the gas/vapor
phase of the ambient (G) (which will be a mixture of ambient atmosphere and an equilibrium concentration of the liquid vapor). The gaseous phase could also be another (immiscible
) liquid phase. At equilibrium, the chemical potential
in the three phases should be equal. It is convenient to frame the discussion in terms of the interfacial energies. We denote the solid–vapor interfacial energy (see surface energy
) as , the solid–liquid interfacial energy as
, the solid–liquid interfacial energy as  and the liquid–vapor energy (i.e. the surface tension
and the liquid–vapor energy (i.e. the surface tension
) as simply , we can write an equation that must be satisfied in equilibrium (known as the Young Equation):
, we can write an equation that must be satisfied in equilibrium (known as the Young Equation):

where is the equilibrium contact angle.
is the equilibrium contact angle.
The Young equation assumes a perfectly flat surface, and in many cases surface roughness and impurities cause a deviation in the equilibrium contact angle from the contact angle predicted by Young's equation. Even in a perfectly smooth surface a drop will assume a wide spectrum of contact angles between the highest (advancing) contact angle, , and the lowest (receding) contact angle,
, and the lowest (receding) contact angle,  . The equilibrium contact angle (
. The equilibrium contact angle ( ) can be calculated from
) can be calculated from  and
and  as was shown theoretically by Tadmor and confirmed experimentally by Chibowski :
as was shown theoretically by Tadmor and confirmed experimentally by Chibowski :

Where,

and,

The contact angle can also be used to determine an interfacial energy (if other interfacial energies are known). This equation can be rewritten as the Young-Dupre equation:

where is the adhesion energy per unit area of the solid and liquid surfaces when in the medium V.
is the adhesion energy per unit area of the solid and liquid surfaces when in the medium V.
using an optical subsystem to capture the profile of a pure liquid on a solid substrate. The angle formed between the liquid/solid interface and the liquid/vapor interface is the contact angle. Older systems used a microscope optical system with a back light. Current-generation systems employ high resolution cameras and software to capture and analyze the contact angle.
The dynamic sessile drop method : The dynamic sessile drop is similar to the static sessile drop but requires the drop to be modified. A common type of dynamic sessile drop study determines the largest contact angle possible without increasing its solid/liquid interfacial area by adding volume dynamically. This maximum angle is the advancing angle. Volume is removed to produce the smallest possible angle, the receding angle. The difference between the advancing and receding angle is the contact angle hysteresis
.
Dynamic Wilhelmy method : A method for calculating average advancing and receding contact angles on solids of uniform geometry. Both sides of the solid must have the same properties. Wetting force on the solid is measured as the solid is immersed in or withdrawn from a liquid of known surface tension.
Single-fiber Wilhelmy method : Dynamic Wilhelmy method applied to single fibers to measure advancing and receding contact angles.
Powder contact angle method : Enables measurement of average contact angle and sorption speed for powders and other porous materials. Change of weight as a function of time is measured.
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
/vapor
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
interface meets a solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
surface. The contact angle is specific for any given system and is determined by the interactions across the three interfaces. Most often the concept is illustrated with a small liquid droplet resting on a flat horizontal solid surface. The shape of the droplet is determined by the Young-Laplace equation, with the contact angle playing the role of a boundary condition. Contact angle is measured using a contact angle goniometer
Goniometer
A goniometer is an instrument that either measures an angle or allows an object to be rotated to a precise angular position. The term goniometry is derived from two Greek words, gōnia, meaning angle, and metron, meaning measure....
. The contact angle is not limited to a liquid/vapour interface; it is equally applicable to the interface of two liquids.
Typical contact angles
If the molecules of a liquid are strongly attracted to the molecules of a solid (for example water on a strongly hydrophilic solid) then a drop of the liquid will completely spread out on the solid surface, corresponding to a contact angle of 0°. Less strongly hydrophilic solids will have a contact angle up to 90°. On many highly hydrophilic surfaces, water droplets will exhibit contact angles of 0° to 30°.If the solid surface is hydrophobic, the contact angle will be larger than 90°. On highly hydrophobic surfaces the surfaces have water contact angles as high as ~120° on low energy materials e.g. fluorinated surfaces. However, some materials with highly rough surfaces may have a water contact angle greater than 150°. These are called superhydrophobic surfaces. Sometimes the contact angle is measured through the gas instead of through the liquid, which reverses 0 and 180 in the above explanation.
 |
 |
Thermodynamics

Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
equilibrium
Thermodynamic equilibrium
In thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance...
between the three phases
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
: the liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
phase of the droplet (L), the solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
phase of the substrate (S), and the gas/vapor
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
phase of the ambient (G) (which will be a mixture of ambient atmosphere and an equilibrium concentration of the liquid vapor). The gaseous phase could also be another (immiscible
Miscibility
Miscibility is the property of liquids to mix in all proportions, forming a homogeneous solution. In principle, the term applies also to other phases , but the main focus is usually on the solubility of one liquid in another...
) liquid phase. At equilibrium, the chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
in the three phases should be equal. It is convenient to frame the discussion in terms of the interfacial energies. We denote the solid–vapor interfacial energy (see surface energy
Surface energy
Surface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
) as
 , the solid–liquid interfacial energy as
, the solid–liquid interfacial energy as  and the liquid–vapor energy (i.e. the surface tension
and the liquid–vapor energy (i.e. the surface tensionSurface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
) as simply
 , we can write an equation that must be satisfied in equilibrium (known as the Young Equation):
, we can write an equation that must be satisfied in equilibrium (known as the Young Equation):
where
 is the equilibrium contact angle.
is the equilibrium contact angle.The Young equation assumes a perfectly flat surface, and in many cases surface roughness and impurities cause a deviation in the equilibrium contact angle from the contact angle predicted by Young's equation. Even in a perfectly smooth surface a drop will assume a wide spectrum of contact angles between the highest (advancing) contact angle,
 , and the lowest (receding) contact angle,
, and the lowest (receding) contact angle,  . The equilibrium contact angle (
. The equilibrium contact angle ( ) can be calculated from
) can be calculated from  and
and  as was shown theoretically by Tadmor and confirmed experimentally by Chibowski :
as was shown theoretically by Tadmor and confirmed experimentally by Chibowski :
Where,

and,

The contact angle can also be used to determine an interfacial energy (if other interfacial energies are known). This equation can be rewritten as the Young-Dupre equation:

where
 is the adhesion energy per unit area of the solid and liquid surfaces when in the medium V.
is the adhesion energy per unit area of the solid and liquid surfaces when in the medium V.Measuring methods
The static sessile drop method : The sessile drop method is measured by a contact angle goniometerGoniometer
A goniometer is an instrument that either measures an angle or allows an object to be rotated to a precise angular position. The term goniometry is derived from two Greek words, gōnia, meaning angle, and metron, meaning measure....
using an optical subsystem to capture the profile of a pure liquid on a solid substrate. The angle formed between the liquid/solid interface and the liquid/vapor interface is the contact angle. Older systems used a microscope optical system with a back light. Current-generation systems employ high resolution cameras and software to capture and analyze the contact angle.
The dynamic sessile drop method : The dynamic sessile drop is similar to the static sessile drop but requires the drop to be modified. A common type of dynamic sessile drop study determines the largest contact angle possible without increasing its solid/liquid interfacial area by adding volume dynamically. This maximum angle is the advancing angle. Volume is removed to produce the smallest possible angle, the receding angle. The difference between the advancing and receding angle is the contact angle hysteresis
Hysteresis
Hysteresis is the dependence of a system not just on its current environment but also on its past. This dependence arises because the system can be in more than one internal state. To predict its future evolution, either its internal state or its history must be known. If a given input alternately...
.
Dynamic Wilhelmy method : A method for calculating average advancing and receding contact angles on solids of uniform geometry. Both sides of the solid must have the same properties. Wetting force on the solid is measured as the solid is immersed in or withdrawn from a liquid of known surface tension.
Single-fiber Wilhelmy method : Dynamic Wilhelmy method applied to single fibers to measure advancing and receding contact angles.
Powder contact angle method : Enables measurement of average contact angle and sorption speed for powders and other porous materials. Change of weight as a function of time is measured.
See also
- GoniometerGoniometerA goniometer is an instrument that either measures an angle or allows an object to be rotated to a precise angular position. The term goniometry is derived from two Greek words, gōnia, meaning angle, and metron, meaning measure....
- MeniscusMeniscusThe meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
- PorosimetryPorosimetryPorosimetry is an analytical technique used to determine various quantifiable aspects of a material's porous nature, such as pore diameter, total pore volume, surface area, and bulk and absolute densities....
- Sessile drop technique
- Surface tensionSurface tensionSurface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
- WettingWettingWetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. The degree of wetting is determined by a force balance between adhesive and cohesive forces.Wetting is important in the bonding or adherence of...
Further reading
- Jacob IsraelachviliJacob IsraelachviliJacob Israelachvili is a professor of chemical engineering and materials at the University of California, Santa Barbara . Israelachvili received his Ph.D. in Physics from Christ's College, Cambridge in 1972, and joined UCSB in 1986. His research has involved study of molecular and interfacial forces...
, Intermolecular and Surface Forces, Academic Press (1985–2004) - D.W. Van Krevelen, Properties of Polymers, 2nd revised edition, Elsevier Scientific Publishing Company, Amsterdam-Oxford-New York (1976)

