
Sulfiredoxin
Encyclopedia
In enzymology, a sulfiredoxin is an enzyme
that catalyzes
the chemical reaction
The 3 substrates
of this enzyme are peroxiredoxin-(S-hydroxy-S-oxocysteine), ATP
, and a thiol
, whereas its 4 products
are peroxiredoxin-(S-hydroxycysteine), ADP
, phosphate
, and a disulfide
.
This enzyme is involved in antioxidant
metabolism by re-activating peroxiredoxin
s, which are a group of peroxidases, when these enzymes are inhibited by over-oxidation.
This enzyme belongs to the family of oxidoreductase
s, specifically those acting on a sulfur group of donors with other, known, acceptors. The systematic name of this enzyme class is peroxiredoxin-(S-hydroxy-S-oxocysteine):thiol oxidoreductase [ATP-hydrolysing; peroxiredoxin
-(S-hydroxycysteine)-forming]. Other names in common use include Srx1, sulphiredoxin, and peroxiredoxin-(S-hydroxy-S-oxocysteine) reductase.
can exist in several different oxidation state
s. The most reduced of these is as a thiol group (Cys-SH). Oxidation of cysteine produces cystine
, which is one half of a disulfide bond
(Cys-S-S-Cys). These lower oxidation states of cysteine (disulfides) are readily reversible, but higher oxidation states, such as sulfinic acid (Cys-SOOH), were once considered irreversible, biologically speaking. This view changed with the discovery of sulfiredoxin, an enzyme that can reduce sulfinic acid back to thiol, in an ATP-dependent manner. Additional work suggests that it plays a role in resolving mixed disulfide bonds.
Initially discovered in yeast, sulfiredoxin is conserved in all eukaryotes, including mammals. In a perfect example of how multiple gene names can confuse the field, sulfiredoxin (Srxn1) was already known as a gene of unknown function, cloned by differential display of an in vitro model of tumorgenesis, and termed “Neoplastic progression 3/Npn3” although nothing about its actual function was reported. As a result, in most mouse microarray studies, sulfiredoxin is termed neoplastic progression 3, and typically classified as “cancer related” or “other” rather than as “antioxidant”.
Npn3/Srxn1 is upregulated by an exceptionally large fold-magnitude in microarray studies of oxidative stress. Npn3/Srxn1 is induced up to 32-fold by D3T (liver), 12-fold by CdCl2, (liver), 4- to 10-fold by parcetamol (liver) and 3.3-flold by paraquat (heart). A survey of the GEO database also indicates a large induction of Npn3/Srxn1 is observed in injury to the lung by hyperoxia (data set GDS247, ID# 102780_at) or phosgene (GDS1244, 1451680_at). That Npn3 and Sxrn1 are synonyms of the same gene has not been pointed out in any of the 15 papers written on Srxn1 since its discovery.
Because it was discovered so recently, the function of sulfiredoxin is not yet fully known and because no knockout of sulfiredoxin in mice is yet available, its true physiological importance remains to be established.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- peroxiredoxin-(S-hydroxy-S-oxocysteine) + ATP + 2 R-SH
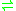 peroxiredoxin-(S-hydroxycysteine) + ADP + phosphate + R-S-S-R
peroxiredoxin-(S-hydroxycysteine) + ADP + phosphate + R-S-S-R
The 3 substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are peroxiredoxin-(S-hydroxy-S-oxocysteine), ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, and a thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
, whereas its 4 products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are peroxiredoxin-(S-hydroxycysteine), ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
, phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
, and a disulfide
Disulfide
In chemistry, a disulfide usually refers to the structural unit composed of a linked pair of sulfur atoms. Disulfide usually refer to a chemical compound that contains a disulfide bond, such as diphenyl disulfide, C6H5S-SC6H5....
.
This enzyme is involved in antioxidant
Antioxidant
An antioxidant is a molecule capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start chain reactions. When...
metabolism by re-activating peroxiredoxin
Peroxiredoxin
Peroxiredoxins are a ubiquitous family of antioxidant enzymes that also control cytokine-induced peroxide levels and thereby mediate signal transduction in mammalian cells.- Classification :...
s, which are a group of peroxidases, when these enzymes are inhibited by over-oxidation.
This enzyme belongs to the family of oxidoreductase
Oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule to another...
s, specifically those acting on a sulfur group of donors with other, known, acceptors. The systematic name of this enzyme class is peroxiredoxin-(S-hydroxy-S-oxocysteine):thiol oxidoreductase [ATP-hydrolysing; peroxiredoxin
Peroxiredoxin
Peroxiredoxins are a ubiquitous family of antioxidant enzymes that also control cytokine-induced peroxide levels and thereby mediate signal transduction in mammalian cells.- Classification :...
-(S-hydroxycysteine)-forming]. Other names in common use include Srx1, sulphiredoxin, and peroxiredoxin-(S-hydroxy-S-oxocysteine) reductase.
Function
The sulfur atom in the side-chain of the amino acid cysteineCysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
can exist in several different oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
s. The most reduced of these is as a thiol group (Cys-SH). Oxidation of cysteine produces cystine
Cystine
Cystine is a dimeric amino acid formed by the oxidation of two cysteine residues that covalently link to make a disulfide bond. This organosulfur compound has the formula 2. It is a white solid, and melts at 247-249 °C...
, which is one half of a disulfide bond
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
(Cys-S-S-Cys). These lower oxidation states of cysteine (disulfides) are readily reversible, but higher oxidation states, such as sulfinic acid (Cys-SOOH), were once considered irreversible, biologically speaking. This view changed with the discovery of sulfiredoxin, an enzyme that can reduce sulfinic acid back to thiol, in an ATP-dependent manner. Additional work suggests that it plays a role in resolving mixed disulfide bonds.
Initially discovered in yeast, sulfiredoxin is conserved in all eukaryotes, including mammals. In a perfect example of how multiple gene names can confuse the field, sulfiredoxin (Srxn1) was already known as a gene of unknown function, cloned by differential display of an in vitro model of tumorgenesis, and termed “Neoplastic progression 3/Npn3” although nothing about its actual function was reported. As a result, in most mouse microarray studies, sulfiredoxin is termed neoplastic progression 3, and typically classified as “cancer related” or “other” rather than as “antioxidant”.
Npn3/Srxn1 is upregulated by an exceptionally large fold-magnitude in microarray studies of oxidative stress. Npn3/Srxn1 is induced up to 32-fold by D3T (liver), 12-fold by CdCl2, (liver), 4- to 10-fold by parcetamol (liver) and 3.3-flold by paraquat (heart). A survey of the GEO database also indicates a large induction of Npn3/Srxn1 is observed in injury to the lung by hyperoxia (data set GDS247, ID# 102780_at) or phosgene (GDS1244, 1451680_at). That Npn3 and Sxrn1 are synonyms of the same gene has not been pointed out in any of the 15 papers written on Srxn1 since its discovery.
Because it was discovered so recently, the function of sulfiredoxin is not yet fully known and because no knockout of sulfiredoxin in mice is yet available, its true physiological importance remains to be established.

