Stork enamine alkylation
Encyclopedia
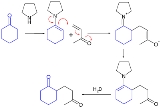
Stork enamine alkylation, also known as the Stork-Enamine reaction, involves the addition of an enamine
to an alpha, beta-unsaturated carbonyl acceptor
in a process similar to the Michael reaction
. The product is then hydrolyzed
by an aqueous acid
to produce a 1,5-dicarbonyl compound.
The process:
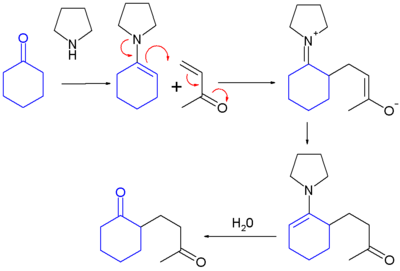 When the electrophile is an acyl halide
When the electrophile is an acyl halide
, a 1,3-diketone
is formed (Stork acylation) . The reaction is named after its inventor: Gilbert Stork
.
s or aldehyde
s with alkyl halides as less reactive electrophile
s :
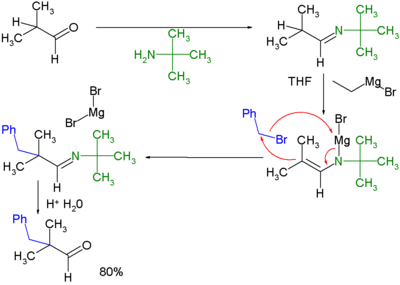 In this method a carbonyl compound is converted to an imine
In this method a carbonyl compound is converted to an imine
by alkylimino-de-oxo-bisubstitution
with a primary amine
. The imine is then reacted with an Grignard reagent to the corresponding magnesium salt to an intermediate capable of displacing a halide. Hydrolysis
once again yields the alkylated ketone.
Enamine
An enamine is an unsaturated compound derived by the reaction of an aldehyde or ketone with a secondary amine followed by loss of H2O.The word "enamine" is derived from the affix en-, used as the suffix of alkene, and the root amine. This can be compared with enol, which is a functional group...
to an alpha, beta-unsaturated carbonyl acceptor
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
in a process similar to the Michael reaction
Michael reaction
The Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile to an alpha, beta unsaturated carbonyl compound. It belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C-C bonds...
. The product is then hydrolyzed
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
by an aqueous acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
to produce a 1,5-dicarbonyl compound.
The process:
- formation of an enamine from a ketoneKetoneIn organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
- addition of the enamine to an alpha, beta-unsaturated aldehydeAldehydeAn aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
or ketone - hydrolysis of the enamine back to a ketone

Acyl halide
An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group....
, a 1,3-diketone
Diketone
A diketone is a molecule containing two ketone groups. The simpliest diketone is diacetyl, also known as 2,3-butanedione. Diacetyl, acetylacetone, and hexane-2,5-dione are examples of 1,2-, 1,3-, and 1,4-diketones, respectively...
is formed (Stork acylation) . The reaction is named after its inventor: Gilbert Stork
Gilbert Stork
Gilbert Stork is a U.S. organic chemist. He is the Eugene Higgins Professor of Chemistry Emeritus at Columbia University. The Stork enamine synthesis is named in his honor.-Education:...
.
Variations
In a special case of this reaction type it is also possible to alkylate ketoneKetone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s or aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s with alkyl halides as less reactive electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s :

Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
by alkylimino-de-oxo-bisubstitution
Alkylimino-de-oxo-bisubstitution
Alkylimino-de-oxo-bisubstitution in organic chemistry is the organic reaction of carbonyl compounds with amines to imines . The reaction name is based on the IUPAC Nomenclature for Transformations...
with a primary amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
. The imine is then reacted with an Grignard reagent to the corresponding magnesium salt to an intermediate capable of displacing a halide. Hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
once again yields the alkylated ketone.

