Sodium phosphide
Encyclopedia
Sodium phosphide, Na3P, is a black, ionic salt containing the alkali metal
sodium
and the phosphide
anion. Na3P is a source of the highly reactive phosphide anion. It should not be confused with sodium phosphate, Na3PO4.
In addition to Na3P, five other binary compositions of sodium and phosphorus are known: NaP, Na3P7, Na3P11, NaP7, and NaP15.
, with a lone pair of electrons on the central phosphorus atom.
prepared sodium phosphide by reacting molten sodium with phosphorus pentachloride.
Many different routes to Na3P have been described. Due to its flammability and toxicity, Na3P (and related salts) are generally prepared in situ
. White phosphorus is reduced by sodium-potassium alloy to give the phosphide salt.
The conversion of white phosphorus
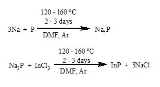
to the phosphide has been well studied. Phosphorus reacts with sodium in an autoclave at 150 °C for 5 hours to produce Na3P.
Alternatively the reaction can be conducted at normal pressures but using a temperatures gradient to generate nonvolatile NaxP phases (x < 3) that then react further with sodium. In some cases, an electron-transfer agent, such as naphthalene
, is used. In such applications, the naphthalene forms the soluble radical
anions and more rapidly reduces the phosphorus.
The trimethylsilyl
derivative is volatile (b.p. 30-35 C @ 0.001 mm Hg) and soluble. It serves as a soluble equivalent to "P3-".
Indium phosphide, a semiconductor arises by treating in-situ generated "sodium phosphide" with indium(III) chloride
. In this process, the phosphide reagent is generated in situ from sodium metal and white phosphorus in N,N’-dimethylformamide, DMF
.
Sodium phosphide is also employed commercially as a catalyst in conjunction with zinc phosphide and aluminium phosphide
for polymer
production. When Na3P is removed from the ternary catalyst polymerization
of propylene
and 4-methyl-1-pentene is not effective.
upon hydrolysis, a process that is so exothermic that fires result. The USDOT has forbidden the transportation of Na3P on passenger aircraft, cargo only aircraft, and trains due to the potential fire and toxic hazards.http://environmentalchemistry.com/yogi/chemicals/cn/Sodium%A0phosphide.html
Alkali metal
The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
and the phosphide
Phosphide
In chemistry, a phosphide is a compound of phosphorus with a less electronegative element or elements. Binary compounds are formed with the majority of less electronegative elements with the exception of Hg, Pb, Sb, Bi, Te, Po...
anion. Na3P is a source of the highly reactive phosphide anion. It should not be confused with sodium phosphate, Na3PO4.
In addition to Na3P, five other binary compositions of sodium and phosphorus are known: NaP, Na3P7, Na3P11, NaP7, and NaP15.
Properties
Like K3P, solid Na3P features pentacoordinate P centers. In the gas phase, molecular Na3P and Li3P are both trigonal pyramidalTrigonal pyramid (chemistry)
In chemistry, a trigonal pyramid is a molecular geometry with one atom at the apex and three atoms at the corners of a trigonal base. When all three atoms at the corners are identical, the molecule belongs to point group C3v. One example of a molecule with a trigonal pyramidal geometry is ammonia...
, with a lone pair of electrons on the central phosphorus atom.
Preparation
The first preparation of Na3P was first reported in the mid-19th century. French researcher, Alexandre BaudrimontAlexandre Baudrimont
Alexandre Edouard Baudrimont was a 19th-century French professor of chemistry who published various books connected to the sciences, languages and the Basque Country :...
prepared sodium phosphide by reacting molten sodium with phosphorus pentachloride.
- 8 Na(l)SodiumSodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
+ PCl5 → 5NaClSodium chlorideSodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
+ Na3P
Many different routes to Na3P have been described. Due to its flammability and toxicity, Na3P (and related salts) are generally prepared in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
. White phosphorus is reduced by sodium-potassium alloy to give the phosphide salt.
The conversion of white phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
to the phosphide has been well studied. Phosphorus reacts with sodium in an autoclave at 150 °C for 5 hours to produce Na3P.
- P4 + 12 Na → 4 Na3P
Alternatively the reaction can be conducted at normal pressures but using a temperatures gradient to generate nonvolatile NaxP phases (x < 3) that then react further with sodium. In some cases, an electron-transfer agent, such as naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
, is used. In such applications, the naphthalene forms the soluble radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
anions and more rapidly reduces the phosphorus.
Uses
Sodium phosphide is a source of the highly reactive phosphide anion. The material is insoluble in all solvents but reacts as a slurry with acids and related electrophiles to give derivatives of the type PM3:- Na3P + 3 M+5 → M3P (M = H, Me3Si)
The trimethylsilyl
Trimethylsilyl
A trimethylsilyl group is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si3], which is in turn bonded to the rest of a molecule...
derivative is volatile (b.p. 30-35 C @ 0.001 mm Hg) and soluble. It serves as a soluble equivalent to "P3-".
Indium phosphide, a semiconductor arises by treating in-situ generated "sodium phosphide" with indium(III) chloride
Indium(III) chloride
Indium chloride is the chemical compound with the formula InCl3. This colorless salt finds some use in organic synthesis as a Lewis acid. It is also the most available soluble derivative of indium.-Synthesis and structure:...
. In this process, the phosphide reagent is generated in situ from sodium metal and white phosphorus in N,N’-dimethylformamide, DMF
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
.

Sodium phosphide is also employed commercially as a catalyst in conjunction with zinc phosphide and aluminium phosphide
Aluminium phosphide
Aluminium phosphide is an inorganic compound used as a wide band gap semiconductor and a fumigant. This colourless solid is generally sold as a grey-green-yellow powder due to the presence of impurities arising from hydrolysis and oxidation.-Properties:...
for polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
production. When Na3P is removed from the ternary catalyst polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
of propylene
Propylene
Propene, also known as propylene or methylethylene, is an unsaturated organic compound having the chemical formula C3H6. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons, and it is also second in natural abundance.-Properties:At room temperature and...
and 4-methyl-1-pentene is not effective.

Precautions
Sodium phosphide is highly dangerous releasing toxic phosphinePhosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
upon hydrolysis, a process that is so exothermic that fires result. The USDOT has forbidden the transportation of Na3P on passenger aircraft, cargo only aircraft, and trains due to the potential fire and toxic hazards.http://environmentalchemistry.com/yogi/chemicals/cn/Sodium%A0phosphide.html

