
Ribonuclease A
Encyclopedia
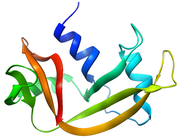
Pancreatic ribonuclease
Pancreatic ribonucleasea are pyrimidine-specific endonucleases found in high quantity in the pancreas of certain mammals and of some reptiles....
that cleaves single-stranded RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
. Bovine pancreatic RNase A is one of the classic model systems of protein science.
History
The importance of bovine pancreatic RNase A was secured when the Armour & Co.Armour and Company
Armour & Company was an American slaughterhouse and meatpacking company founded in Chicago, Illinois, in 1867 by the Armour brothers, led by Philip Danforth Armour. By 1880, the company was Chicago's most important business and helped make the city and its Union Stock Yards the center of the...
(of hot dog
Hot dog
A hot dog is a sausage served in a sliced bun. It is very often garnished with mustard, ketchup, onions, mayonnaise, relish and/or sauerkraut.-History:...
fame) purified a kilogram of it, and gave 10 mg samples away free to any interested scientists. The ability to have a single lot of purified enzyme instantly made RNase a predominant model system for protein studies.
RNase A was the model protein used to work out many spectroscopic methods for assaying protein structure, including absorbance, circular dichroism/optical rotary dispersion, Raman, EPR and NMR spectroscopy. RNase A was also the first model protein for the development of several chemical structural methods, such as limited proteolysis of disordered segments, chemical modification of exposed side chains, and antigenic recognition.
Ribonuclease-S, which is RNase A that has been treated with subtilisin
Subtilisin
Subtilisin is a non-specific protease initially obtained from Bacillus subtilis.Subtilisins belong to subtilases, a group of serine proteases that initiate the nucleophilic attack on the peptide bond through a serine residue at the active site. They are physically and chemically...
, was the third protein to have its structure solved, in 1967.
Studies of the oxidative folding
Oxidative folding
Oxidative protein folding is a process that is responsible for the formation of disulfide bonds between cysteine residues in proteins. The driving force behind this process is a redox reaction, in which electrons are passed between several proteins and finally to a terminal electron acceptor.- In...
of RNase A led Chris Anfinsen
Christian B. Anfinsen
Christian Boehmer Anfinsen, Jr. was an American biochemist. He shared the 1972 Nobel Prize in Chemistry with Stanford Moore and William Howard Stein for work on ribonuclease, especially concerning the connection between the amino acid sequence and the biologically active conformation...
to enunciate the
thermodynamic hypothesis of protein folding, which states that the folded form of a protein represents the minimum of its free energy.
RNase A was the first protein for showing the effects of non-native isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s of X-Pro peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
s in protein folding.
RNase A was the first protein to be studied by multiple sequence alignment
Multiple sequence alignment
A multiple sequence alignment is a sequence alignment of three or more biological sequences, generally protein, DNA, or RNA. In many cases, the input set of query sequences are assumed to have an evolutionary relationship by which they share a lineage and are descended from a common ancestor...
and by comparing the properties of evolutionarily related proteins.
Structure and Properties
RNase A is a relatively small protein (124 residues, ~13.7 kDa). It can be characterized as a two-layer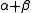 protein that is folded in half to resemble a taco
protein that is folded in half to resemble a tacoTaco
A taco is a traditional Mexican dish composed of a corn or wheat tortilla folded or rolled around a filling. A taco can be made with a variety of fillings, including beef, chicken, seafood, vegetables and cheese, allowing for great versatility and variety...
, with a deep cleft for binding the RNA substrate. The first layer is composed of three alpha helices
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
(residues 3-13, 24-34 and 50-60) from the N-terminal half of the protein. The second layer consist of three β-hairpins
Beta hairpin
The beta hairpin structural motif is the simplest protein motif involving two beta strands that look like a hairpin. The motif consists of two strands that are adjacent in primary structure oriented in an antiparallel arrangement and linked by a short loop of two to five amino acids...
(residues 61-74, 79-104 and 105-124 from the C-terminal half) arranged in two β-sheets
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
. The hairpins 61-74 and 105-124 form a four-stranded, antiparallel β-sheet that lies on helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
3 (residues 50-60). The longest β-hairpin 79-104 mates with a short β-strand (residues 42-45) to form a three-stranded, antiparallel β-sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
that lies on helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
2 (residues 24-34).
RNase A has four disulfide bonds in its native state: Cys26-Cys84, Cys58-110, Cys40-95 and Cys65-72. The first two (26-84 and 58-110) are essential for conformational folding; each joins an alpha helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
of the first layer to a beta sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
of the second layer, forming a small hydrophobic core in its vicinity. The latter two disulfide bonds (40-95 and 65-72) are less essential for folding; either one can be reduced (but not both) without affecting the native structure under physiological conditions. These disulfide bonds connect loop segments and are relatively exposed to solvent. Interestingly, the 65-72 disulfide bond has an extraordinarily high propensity to form, significantly more than would be expected from its loop entropy, both as a peptide and in the full-length protein. This suggests that the 61-74 β-hairpin has a high propensity to fold conformationally.
RNase A is a basic protein (pI =9.63); its many positive charges are consistent with its binding to RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
(a poly-anion). More generally, RNase A is unusually polar or, rather, unusually lacking in hydrophobic groups, especially aliphatic ones. This may account for its need of four disulfide bonds to stabilize its structure.
The low hydrophobic content may also serve to reduce the physical repulsion between highly charged groups (its own and those of its substrate RNA) and regions of low dielectric constant
Dielectric constant
The relative permittivity of a material under given conditions reflects the extent to which it concentrates electrostatic lines of flux. In technical terms, it is the ratio of the amount of electrical energy stored in a material by an applied voltage, relative to that stored in a vacuum...
(the nonpolar residues).
The N-terminal α-helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
of RNase A (residues 3-13) is connected to the rest of RNase A by a flexible linker (residues 16-23). As shown by F. M. Richards, this linker may be cleaved by subtilisin
Subtilisin
Subtilisin is a non-specific protease initially obtained from Bacillus subtilis.Subtilisins belong to subtilases, a group of serine proteases that initiate the nucleophilic attack on the peptide bond through a serine residue at the active site. They are physically and chemically...
between residues 20 and 21 without causing the N-terminal helix to dissociate from the rest of RNase A. The peptide-protein complex is called RNase S, the peptide (residues 1-20) is called the S-peptide and the remainder (residues 21-124) is called the S-protein. The dissociation constant
Dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into...
of the S-peptide for the S-protein is roughly 30 pM; this tight binding can be exploited for protein purification
Protein purification
Protein purification is a series of processes intended to isolate a single type of protein from a complex mixture. Protein purification is vital for the characterization of the function, structure and interactions of the protein of interest. The starting material is usually a biological tissue or...
by attaching the S-peptide to the protein of interest and passing a mixture over an affinity column with bound S-protein. [A smaller C-peptide
C-peptide
C-peptide is a protein that is produced in the body along with insulin. First preproinsulin is secreted with an A-chain, C-peptide, a B-chain, and a signal sequence. The signal sequence is cut off, leaving proinsulin...
(residues 1-13) also works.] The RNase S model system has also been used for studying protein folding by coupling folding and association. The S-peptide was the first peptide from a native protein shown to have (flickering) secondary structure in isolation (by Klee and Brown in 1967).
RNase A cleaves specifically after pyrimidine
Pyrimidine
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
nucleotides. Cleavage takes place in two steps: first, the 3’,5’-phosphodiester bond is cleaved to generate a 2’,3’-cyclic phosphodiester intermediate; second, the cyclic phosphodiester is hydrolyzed to a 3’-monophosphate. It can be inhibited by ribonuclease inhibitor
Ribonuclease inhibitor
Ribonuclease inhibitor is a large , acidic , leucine-rich repeat protein that forms extremely tight complexes with certain ribonucleases. It is a major cellular protein, comprising ~0.1% of all cellular protein by weight, and appears to play an important role in regulating the lifetime of RNA.RI...
protein, by heavy metal ions, and by uridine-vanadate complexes.
Enzymatic Mechanism
The positive charges of RNase A lie mainly in a deep cleft between two lobes. The RNA substrate lies in this cleft and is cleaved by two catalytic histidineHistidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
s, His12 and His119, via a 2'-3' cyclic phosphate intermediate that is stabilized by nearby lysines such as Lys7, Lys41 and Lys66.
Anti-cancer effects
RNase A, and to a greater extent its oligomers and some homologs (such as onconase from frogs), have cytotoxic and cytostatic effects, particularly on cancer cells. This has led to the development of onconase as a cancer therapeutic, particularly for external use against skin cancers. As with many protein drugs, the internal use of non-human ribonucleases such as onconase is limited by the patient's immune response.Other biological effects
Ribonuclease is also related to angiogeninAngiogenin
Angiogenin also known as ribonuclease 5 is a protein that in humans is encoded by the ANG gene. Angiogenin is a potent stimulator of new blood vessel formation...
, which is involved in blood vessel
Blood vessel
The blood vessels are the part of the circulatory system that transports blood throughout the body. There are three major types of blood vessels: the arteries, which carry the blood away from the heart; the capillaries, which enable the actual exchange of water and chemicals between the blood and...
development
Developmental biology
Developmental biology is the study of the process by which organisms grow and develop. Modern developmental biology studies the genetic control of cell growth, differentiation and "morphogenesis", which is the process that gives rise to tissues, organs and anatomy.- Related fields of study...
.

