
Chemical affinity
Encyclopedia
In chemical physics
and physical chemistry
, chemical affinity is the electronic property by which dissimilar chemical species
are capable of forming chemical compounds. Chemical affinity can also refer to the tendency of an atom
or compound to combine by chemical reaction
with atoms or compounds of unlike composition.
According to chemistry historian Henry Leicester, the influential 1923 textbook Thermodynamics and the Free Energy of Chemical Reactions by Gilbert N. Lewis
and Merle Randall
led to the replacement of the term "affinity" by the term "free energy
" in much of the English-speaking world.
Ilya Prigogine
summarized the concept of affinity, saying,
of Gibbs free energy
G with respect to extent of reaction ξ at constant pressure
and temperature
. That is,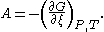
It follows that affinity is positive for spontaneous reactions
.
In 1923, the Belgian mathematician and physicist Théophile de Donder
derived a relation between affinity and the Gibbs free energy of a chemical reaction
. Through a series of derivations, de Donder showed that if we consider a mixture of chemical species
with the possibility of chemical reaction
, it can be proven that the following relation holds:
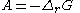
With the writings of Théophile de Donder
as precedent, Ilya Prigogine
and Defay in Chemical Thermodynamics (1954) defined chemical affinity in terms of the uncompensated heat
of reaction Q the reaction progress variable
or reaction extent ξ; as the ratio of their infinitesimal
increments
:
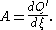
This definition is useful for quantifying the factors responsible both for the state of equilibrium systems (where ), and for changes of state of non-equilibrium systems (where A ≠ 0).
s. A broad definition, used generally throughout history, is that chemical affinity is that whereby substances enter into or resist decomposition. .
The term affinity has been used figuratively since c. 1600 in discussions of structural relationships in chemistry, philology
, etc., and reference to "natural attraction" is from 1616.
The idea of affinity is extremely old. Many attempts have been made at identifying its origins. The majority of such attempts, however, except in a general manner, end in futility since "affinities" lie at the basis of all magic
, thereby pre-dating science
. Physical chemistry
, however, was one of the first branches of science to study and formulate a "theory of affinity". The name affinitas was first used in the sense of chemical relation by German philosopher Albertus Magnus
near the year 1250. Later, those as Robert Boyle
, John Mayow
, Johann Glauber, Isaac Newton
, and Georg Stahl put forward ideas on elective affinity in attempts to explain how heat
is evolved during combustion reactions.
The modern term chemical affinity is a somewhat modified variation of its eighteenth-century precursor "elective affinity" or elective attractions, a coinage of the Swedish chemist Torbern Olof Bergman from his book De attractionibus electivis (1775). Antoine Lavoisier
, in his famed 1789 Traité Élémentaire de Chimie
(Elements of Chemistry), refers to Bergmann’s work and discusses the concept of elective affinities or attractions.
Goethe used the concept in his novel Elective Affinities
, (1809)
. Geoffroy's name is best known in connection with these tables of "affinities" (tables des rapports), which were first presented to the French Academy in 1718 and 1720, as shown below:
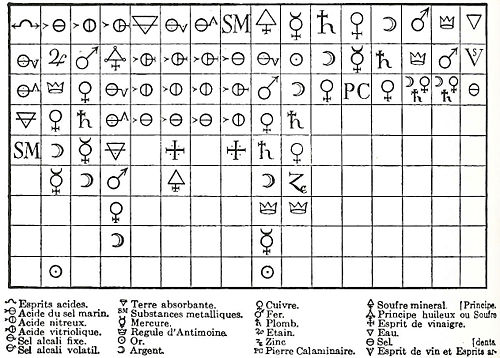
These were lists, prepared by collating observations on the actions of substances one upon another, showing the varying degrees of affinity exhibited by analogous bodies for different reagent
s, and they retained their vogue for the rest of the century, until displaced by the profounder conceptions introduced by Claude Berthollet
.
Chemical physics
Chemical physics is a subdiscipline of chemistry and physics that investigates physicochemical phenomena using techniques from atomic and molecular physics and condensed matter physics; it is the branch of physics that studies chemical processes from the point of view of physics...
and physical chemistry
Physical chemistry
Physical chemistry is the study of macroscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of physical laws and concepts...
, chemical affinity is the electronic property by which dissimilar chemical species
Chemical species
Chemical species are atoms, molecules, molecular fragments, ions, etc., being subjected to a chemical process or to a measurement. Generally, a chemical species can be defined as an ensemble of chemically identical molecular entities that can explore the same set of molecular energy levels on a...
are capable of forming chemical compounds. Chemical affinity can also refer to the tendency of an atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
or compound to combine by chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
with atoms or compounds of unlike composition.
According to chemistry historian Henry Leicester, the influential 1923 textbook Thermodynamics and the Free Energy of Chemical Reactions by Gilbert N. Lewis
Gilbert N. Lewis
Gilbert Newton Lewis was an American physical chemist known for the discovery of the covalent bond , his purification of heavy water, his reformulation of chemical thermodynamics in a mathematically rigorous manner accessible to ordinary chemists, his theory of Lewis acids and...
and Merle Randall
Merle Randall
Merle Randall was an American physical chemist famous for his work, over the period of 25 years, in measuring free energy calculations of compounds with Gilbert N. Lewis...
led to the replacement of the term "affinity" by the term "free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
" in much of the English-speaking world.
Modern conceptions
In modern terms, we relate affinity to the phenomenon whereby certain atoms or molecules have the tendency to aggregate or bond. For example, in the 1919 book Chemistry of Human Life physician George W. Carey states that, "Health depends on a proper amount of iron phosphate Fe3(PO4)2 in the blood, for the molecules of this salt have chemical affinity for oxygen and carry it to all parts of the organism." In this antiquated context, chemical affinity is sometimes found synonymous with the term "magnetic attraction". Many writings, up until about 1925, also refer to a "law of chemical affinity".Ilya Prigogine
Ilya Prigogine
Ilya, Viscount Prigogine was a Russian-born naturalized Belgian physical chemist and Nobel Laureate noted for his work on dissipative structures, complex systems, and irreversibility.-Biography :...
summarized the concept of affinity, saying,
Thermodynamics
The present IUPAC definition is that affinity A is the negative partial derivativePartial derivative
In mathematics, a partial derivative of a function of several variables is its derivative with respect to one of those variables, with the others held constant...
of Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
G with respect to extent of reaction ξ at constant pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
and temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
. That is,
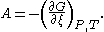
It follows that affinity is positive for spontaneous reactions
Spontaneous process
A spontaneous process is the time-evolution of a system in which it releases free energy and moves to a lower, more thermodynamically stable energy state...
.
In 1923, the Belgian mathematician and physicist Théophile de Donder
Théophile de Donder
Théophile Ernest de Donder was a Belgian mathematician and physicist famous for his 1923 work in developing correlations between the Newtonian concept of chemical affinity and the Gibbsian concept of free energy.-Education:...
derived a relation between affinity and the Gibbs free energy of a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
. Through a series of derivations, de Donder showed that if we consider a mixture of chemical species
Chemical species
Chemical species are atoms, molecules, molecular fragments, ions, etc., being subjected to a chemical process or to a measurement. Generally, a chemical species can be defined as an ensemble of chemically identical molecular entities that can explore the same set of molecular energy levels on a...
with the possibility of chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
, it can be proven that the following relation holds:
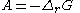
With the writings of Théophile de Donder
Théophile de Donder
Théophile Ernest de Donder was a Belgian mathematician and physicist famous for his 1923 work in developing correlations between the Newtonian concept of chemical affinity and the Gibbsian concept of free energy.-Education:...
as precedent, Ilya Prigogine
Ilya Prigogine
Ilya, Viscount Prigogine was a Russian-born naturalized Belgian physical chemist and Nobel Laureate noted for his work on dissipative structures, complex systems, and irreversibility.-Biography :...
and Defay in Chemical Thermodynamics (1954) defined chemical affinity in terms of the uncompensated heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
of reaction Q the reaction progress variable
Stoichiometry
Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
or reaction extent ξ; as the ratio of their infinitesimal
Infinitesimal
Infinitesimals have been used to express the idea of objects so small that there is no way to see them or to measure them. The word infinitesimal comes from a 17th century Modern Latin coinage infinitesimus, which originally referred to the "infinite-th" item in a series.In common speech, an...
increments
Increment
An increment is an increase of some amount, either fixed or variable. For example one's salary may have a fixed annual increment or one based on a percentage of its current value...
:
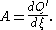
This definition is useful for quantifying the factors responsible both for the state of equilibrium systems (where ), and for changes of state of non-equilibrium systems (where A ≠ 0).
History
"Chemical affinity", historically, refers to the "force" that causes chemical reactionChemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s. A broad definition, used generally throughout history, is that chemical affinity is that whereby substances enter into or resist decomposition. .
The term affinity has been used figuratively since c. 1600 in discussions of structural relationships in chemistry, philology
Philology
Philology is the study of language in written historical sources; it is a combination of literary studies, history and linguistics.Classical philology is the philology of Greek and Classical Latin...
, etc., and reference to "natural attraction" is from 1616.
The idea of affinity is extremely old. Many attempts have been made at identifying its origins. The majority of such attempts, however, except in a general manner, end in futility since "affinities" lie at the basis of all magic
Magic (paranormal)
Magic is the claimed art of manipulating aspects of reality either by supernatural means or through knowledge of occult laws unknown to science. It is in contrast to science, in that science does not accept anything not subject to either direct or indirect observation, and subject to logical...
, thereby pre-dating science
Science
Science is a systematic enterprise that builds and organizes knowledge in the form of testable explanations and predictions about the universe...
. Physical chemistry
Physical chemistry
Physical chemistry is the study of macroscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of physical laws and concepts...
, however, was one of the first branches of science to study and formulate a "theory of affinity". The name affinitas was first used in the sense of chemical relation by German philosopher Albertus Magnus
Albertus Magnus
Albertus Magnus, O.P. , also known as Albert the Great and Albert of Cologne, is a Catholic saint. He was a German Dominican friar and a bishop, who achieved fame for his comprehensive knowledge of and advocacy for the peaceful coexistence of science and religion. Those such as James A. Weisheipl...
near the year 1250. Later, those as Robert Boyle
Robert Boyle
Robert Boyle FRS was a 17th century natural philosopher, chemist, physicist, and inventor, also noted for his writings in theology. He has been variously described as English, Irish, or Anglo-Irish, his father having come to Ireland from England during the time of the English plantations of...
, John Mayow
John Mayow
John Mayow FRS was a chemist, physician, and physiologist who is remembered today for conducting early research into respiration and the nature of air...
, Johann Glauber, Isaac Newton
Isaac Newton
Sir Isaac Newton PRS was an English physicist, mathematician, astronomer, natural philosopher, alchemist, and theologian, who has been "considered by many to be the greatest and most influential scientist who ever lived."...
, and Georg Stahl put forward ideas on elective affinity in attempts to explain how heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
is evolved during combustion reactions.
The modern term chemical affinity is a somewhat modified variation of its eighteenth-century precursor "elective affinity" or elective attractions, a coinage of the Swedish chemist Torbern Olof Bergman from his book De attractionibus electivis (1775). Antoine Lavoisier
Antoine Lavoisier
Antoine-Laurent de Lavoisier , the "father of modern chemistry", was a French nobleman prominent in the histories of chemistry and biology...
, in his famed 1789 Traité Élémentaire de Chimie
Traité Élémentaire de Chimie
Traité élémentaire de chimie is an influential textbook written by Antoine Lavoisier published in 1789 and translated into English by Robert Kerr in 1790.The book is considered to be the first modern chemical textbook...
(Elements of Chemistry), refers to Bergmann’s work and discusses the concept of elective affinities or attractions.
Goethe used the concept in his novel Elective Affinities
Elective Affinities
Elective Affinities , also translated under the title Kindred by Choice, is the third novel by Johann Wolfgang von Goethe, published in 1809. The title is taken from a scientific term once used to describe the tendency of chemical species to combine with certain substances or species in preference...
, (1809)
Geoffroy's 1718 affinity table
The first-ever affinity table, which was based on displacement reactions, was published in 1718 by the French chemist Étienne François GeoffroyÉtienne François Geoffroy
Étienne François Geoffroy , French physician and chemist, best known for his 1718 affinity tables. He first contemplated a career as an apothecary, but then decided to practice medicine. He is sometimes known as Geoffroy the Elder.-Biography:He was born in Paris...
. Geoffroy's name is best known in connection with these tables of "affinities" (tables des rapports), which were first presented to the French Academy in 1718 and 1720, as shown below:

These were lists, prepared by collating observations on the actions of substances one upon another, showing the varying degrees of affinity exhibited by analogous bodies for different reagent
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
s, and they retained their vogue for the rest of the century, until displaced by the profounder conceptions introduced by Claude Berthollet
Claude Louis Berthollet
Claude Louis Berthollet was a Savoyard-French chemist who became vice president of the French Senate in 1804.-Biography:...
.
See also
- ChemistryChemistryChemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
- Chemical reactionChemical reactionA chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- Chemical bondChemical bondA chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
- ElectronegativityElectronegativityElectronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
- Electron affinityElectron affinityThe Electron affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion....
- Étienne François GeoffroyÉtienne François GeoffroyÉtienne François Geoffroy , French physician and chemist, best known for his 1718 affinity tables. He first contemplated a career as an apothecary, but then decided to practice medicine. He is sometimes known as Geoffroy the Elder.-Biography:He was born in Paris...
— Geoffroy's 1718 Affinity Table - Valency
- Affinity chromatographyAffinity chromatographyAffinity chromatography is a method of separating biochemical mixtures and based on a highly specific interaction such as that between antigen and antibody, enzyme and substrate, or receptor and ligand.-Uses:Affinity chromatography can be used to:...
- Affinity electrophoresisAffinity electrophoresisAffinity electrophoresis is a general name for many analytical methods used in biochemistry and biotechnology. Both qualitative and quantitative information may be obtained through affinity electrophoresis. The methods include the so-called mobility shift electrophoresis, charge shift...
External links
- William WhewellWilliam WhewellWilliam Whewell was an English polymath, scientist, Anglican priest, philosopher, theologian, and historian of science. He was Master of Trinity College, Cambridge.-Life and career:Whewell was born in Lancaster...
. "Establishment and Development of the Idea of Chemical Affinity". History of Scientific Ideas. 2:15ff. - Chemical Affinity and Absolute Zero - 1920 Nobel Prize in Chemistry Presentation Speech by Gerard de GeerGerard De GeerBaron Gerard Jacob De Geer was a Swedish geologist who made significant contributions to Quaternary geology, particularly geomorphology and geochronology. De Geer is best known for his discovery of varves.- Early life and family :...
- Elements, Principles and the Narrative of Affinity – Essay Review

