
Oxyhydrogen
Encyclopedia
Oxyhydrogen is a mixture of hydrogen
(H2) and oxygen
(O2) gases, typically in a 2:1 molar ratio, the same proportion as water. This gaseous mixture is used for torches for the processing of refractory
materials and was the first gaseous mixture used for welding
. In practice a ratio of 4:1 or 5:1 hydrogen:oxygen is required to avoid an oxidizing flame.
when brought to its autoignition temperature
. For a stoichiometric mixture at normal atmospheric pressure
, autoignition occurs at about 570 °C (1065 °F). The minimum energy required to ignite such a mixture with a spark is about 20 microjoule
s. At standard temperature and pressure
, oxyhydrogen can burn when it is between about 4% and 95% hydrogen by volume.
When ignited, the gas mixture converts to water vapor
and releases energy
, which sustains the reaction: 241.8 kJ
of energy (LHV) for every mole
of burned. The amount of heat energy released is independent of the mode of combustion, but the temperature of the flame varies. The maximum temperature of about 2800 °C is achieved with a pure stoichiometric
mixture, about 700 degrees hotter than a hydrogen flame in air. When either of the gases are mixed in excess of this ratio, or when mixed with an inert gas
like nitrogen, the heat must spread throughout a greater quantity of matter and the temperature will be lower.
, which uses an electric current
to dissociate the water molecules:
William Nicholson
was the first to decompose water in this manner in 1800. The energy required to generate the oxyhydrogen always exceeds the energy released by combusting it. (See Electrolysis of water#Efficiency).
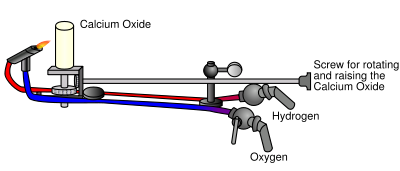
s have been described, such as the limelight
, which used an oxyhydrogen flame to heat a piece of lime
to white hot
incandescence
. Because of the explosiveness of the oxyhydrogen, limelights have been replaced by electric lighting.
Oxyhydrogen was once used in working platinum
because at the time such a torch was the only device that could attain the temperature required to melt the metal (1768.3 °C). These techniques have been superseded by the electric arc furnace
.
was developed by English
mineralogist
Edward Daniel Clarke
and American
chemist
Robert Hare
in the early nineteenth century. It produced a flame hot enough to melt such refractory
materials as platinum
, porcelain
, and fire brick
, and was a valuable tool in several fields of science.
) with oxygen (the oxidizer). It is used for cutting and welding
metal
s, glass
, and thermoplastic
s.
Due to competition from the acetylene-fueled cutting torch and from arc welding, the oxyhydrogen torch is seldom used today, but it remains the preferred cutting tool in some niche applications—see oxy-fuel welding and cutting
.
power supply and an electrolytic cell with a pressure gauge and flashback arrestor
. Water is decomposed on-demand into oxyhydrogen, obviating the need for separate hydrogen and oxygen tanks. The original was designed in 1962 by William Rhodes and Raymond Henes of the Henes Manufacturing Co. (now Arizona Hydrogen Manufacturing, Inc.) and marketed under the trade mark "Water Welder". A hypodermic needle
was originally used for the torch tip.
Oxyhydrogen is also often mentioned in conjunction with devices that claim to operate a vehicle using water as a fuel. The most common and decisive counter-argument against producing this gas on-board to use as a fuel or fuel additive is that the energy required to split water molecules exceeds the energy recouped by burning it.
This should also not be confused with hydrogen-fueled cars where the hydrogen is produced elsewhere and used as fuel or where it is used as as fuel enhancement.
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
(H2) and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
(O2) gases, typically in a 2:1 molar ratio, the same proportion as water. This gaseous mixture is used for torches for the processing of refractory
Refractory
A refractory material is one that retains its strength at high temperatures. ASTM C71 defines refractories as "non-metallic materials having those chemical and physical properties that make them applicable for structures, or as components of systems, that are exposed to environments above...
materials and was the first gaseous mixture used for welding
Welding
Welding is a fabrication or sculptural process that joins materials, usually metals or thermoplastics, by causing coalescence. This is often done by melting the workpieces and adding a filler material to form a pool of molten material that cools to become a strong joint, with pressure sometimes...
. In practice a ratio of 4:1 or 5:1 hydrogen:oxygen is required to avoid an oxidizing flame.
Properties
Oxyhydrogen will combustCombustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
when brought to its autoignition temperature
Autoignition temperature
The autoignition temperature or kindling point of a substance is the lowest temperature at which it will spontaneously ignite in a normal atmosphere without an external source of ignition, such as a flame or spark. This temperature is required to supply the activation energy needed for combustion...
. For a stoichiometric mixture at normal atmospheric pressure
Atmospheric pressure
Atmospheric pressure is the force per unit area exerted into a surface by the weight of air above that surface in the atmosphere of Earth . In most circumstances atmospheric pressure is closely approximated by the hydrostatic pressure caused by the weight of air above the measurement point...
, autoignition occurs at about 570 °C (1065 °F). The minimum energy required to ignite such a mixture with a spark is about 20 microjoule
Joule
The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
s. At standard temperature and pressure
Standard conditions for temperature and pressure
Standard condition for temperature and pressure are standard sets of conditions for experimental measurements established to allow comparisons to be made between different sets of data...
, oxyhydrogen can burn when it is between about 4% and 95% hydrogen by volume.
When ignited, the gas mixture converts to water vapor
Water vapor
Water vapor or water vapour , also aqueous vapor, is the gas phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Under typical atmospheric conditions, water vapor is continuously...
and releases energy
Heat of combustion
The heat of combustion is the energy released as heat when a compound undergoes complete combustion with oxygen under standard conditions. The chemical reaction is typically a hydrocarbon reacting with oxygen to form carbon dioxide, water and heat...
, which sustains the reaction: 241.8 kJ
Joule
The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
of energy (LHV) for every mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
of burned. The amount of heat energy released is independent of the mode of combustion, but the temperature of the flame varies. The maximum temperature of about 2800 °C is achieved with a pure stoichiometric
Stoichiometry
Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
mixture, about 700 degrees hotter than a hydrogen flame in air. When either of the gases are mixed in excess of this ratio, or when mixed with an inert gas
Inert gas
An inert gas is a non-reactive gas used during chemical synthesis, chemical analysis, or preservation of reactive materials. Inert gases are selected for specific settings for which they are functionally inert since the cost of the gas and the cost of purifying the gas are usually a consideration...
like nitrogen, the heat must spread throughout a greater quantity of matter and the temperature will be lower.
Production
A pure stoichiometric mixture may be obtained by water electrolysisElectrolysis of water
Electrolysis of water is the decomposition of water into oxygen and hydrogen gas due to an electric current being passed through the water.-Principle:...
, which uses an electric current
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
to dissociate the water molecules:
- electrolysis: 2 H2O → 2 H2 + O2
- combustion: 2 H2 + O2 → 2 H2O
William Nicholson
William Nicholson (chemist)
William Nicholson was a renowned English chemist and writer on "natural philosophy" and chemistry, as well as a translator, journalist, publisher, scientist, and inventor.-Early life:...
was the first to decompose water in this manner in 1800. The energy required to generate the oxyhydrogen always exceeds the energy released by combusting it. (See Electrolysis of water#Efficiency).
Applications

Lighting
Many forms of oxyhydrogen lampGas lighting
Gas lighting is production of artificial light from combustion of a gaseous fuel, including hydrogen, methane, carbon monoxide, propane, butane, acetylene, ethylene, or natural gas. Before electricity became sufficiently widespread and economical to allow for general public use, gas was the most...
s have been described, such as the limelight
Limelight
Limelight is a type of stage lighting once used in theatres and music halls. An intense illumination is created when an oxyhydrogen flame is directed at a cylinder of quicklime , which can be heated to 2572 °C before melting. The light is produced by a combination of incandescence and...
, which used an oxyhydrogen flame to heat a piece of lime
Calcium oxide
Calcium oxide , commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline crystalline solid at room temperature....
to white hot
Black body
A black body is an idealized physical body that absorbs all incident electromagnetic radiation. Because of this perfect absorptivity at all wavelengths, a black body is also the best possible emitter of thermal radiation, which it radiates incandescently in a characteristic, continuous spectrum...
incandescence
Incandescence
Incandescence is the emission of light from a hot body as a result of its temperature. The term derives from the Latin verb incandescere, to glow white....
. Because of the explosiveness of the oxyhydrogen, limelights have been replaced by electric lighting.
Oxyhydrogen was once used in working platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
because at the time such a torch was the only device that could attain the temperature required to melt the metal (1768.3 °C). These techniques have been superseded by the electric arc furnace
Electric arc furnace
An electric arc furnace is a furnace that heats charged material by means of an electric arc.Arc furnaces range in size from small units of approximately one ton capacity up to about 400 ton units used for secondary steelmaking...
.
Oxyhydrogen blowpipe
The oxy-hydrogen blowpipeBlowpipe (tool)
The term blowpipe refers to one of several tools used to direct streams of gases into any of several working media.- Blowpipes for torches :...
was developed by English
England
England is a country that is part of the United Kingdom. It shares land borders with Scotland to the north and Wales to the west; the Irish Sea is to the north west, the Celtic Sea to the south west, with the North Sea to the east and the English Channel to the south separating it from continental...
mineralogist
Mineralogy
Mineralogy is the study of chemistry, crystal structure, and physical properties of minerals. Specific studies within mineralogy include the processes of mineral origin and formation, classification of minerals, their geographical distribution, as well as their utilization.-History:Early writing...
Edward Daniel Clarke
Edward Daniel Clarke
Edward Daniel Clarke was an English naturalist, mineralogist and traveller.-Life:Edward Daniel Clarke was born at Willingdon, Sussex, and educated first at Tonbridge....
and American
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
chemist
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
Robert Hare
Robert Hare (chemist)
Robert Hare was an early American chemist.Hare was born in Philadelphia, Pennsylvania on January 17, 1781. He developed and experimented with the oxy-hydrogen blowpipe, with Edward Daniel Clarke of Oxford, shortly after 1800. He married Harriett Clark and had six children...
in the early nineteenth century. It produced a flame hot enough to melt such refractory
Refractory
A refractory material is one that retains its strength at high temperatures. ASTM C71 defines refractories as "non-metallic materials having those chemical and physical properties that make them applicable for structures, or as components of systems, that are exposed to environments above...
materials as platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
, porcelain
Porcelain
Porcelain is a ceramic material made by heating raw materials, generally including clay in the form of kaolin, in a kiln to temperatures between and...
, and fire brick
Fire brick
A fire brick, firebrick, or refractory brick is a block of refractory ceramic material used in lining furnaces, kilns, fireboxes, and fireplaces. A refractory brick is built primarily to withstand high temperature, but will also usually have a low thermal conductivity for greater energy efficiency...
, and was a valuable tool in several fields of science.
Oxyhydrogen torch
An oxyhydrogen torch is an oxy-gas torch, which burns hydrogen (the fuelFuel
Fuel is any material that stores energy that can later be extracted to perform mechanical work in a controlled manner. Most fuels used by humans undergo combustion, a redox reaction in which a combustible substance releases energy after it ignites and reacts with the oxygen in the air...
) with oxygen (the oxidizer). It is used for cutting and welding
Welding
Welding is a fabrication or sculptural process that joins materials, usually metals or thermoplastics, by causing coalescence. This is often done by melting the workpieces and adding a filler material to form a pool of molten material that cools to become a strong joint, with pressure sometimes...
metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s, glass
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
, and thermoplastic
Thermoplastic
Thermoplastic, also known as a thermosoftening plastic, is a polymer that turns to a liquid when heated and freezes to a very glassy state when cooled sufficiently...
s.
Due to competition from the acetylene-fueled cutting torch and from arc welding, the oxyhydrogen torch is seldom used today, but it remains the preferred cutting tool in some niche applications—see oxy-fuel welding and cutting
Oxy-fuel welding and cutting
Oxy-fuel welding and oxy-fuel cutting are processes that use fuel gases and oxygen to weld and cut metals, respectively. French engineers Edmond Fouché and Charles Picard became the first to develop oxygen-acetylene welding in 1903...
.
Water torch
A "water torch" is a portable oxyhydrogen torch that combines a DCDirect current
Direct current is the unidirectional flow of electric charge. Direct current is produced by such sources as batteries, thermocouples, solar cells, and commutator-type electric machines of the dynamo type. Direct current may flow in a conductor such as a wire, but can also flow through...
power supply and an electrolytic cell with a pressure gauge and flashback arrestor
Flashback arrestor
A flashback arrestor or flame arrestor is a device most commonly used in oxy-fuel welding and cutting to stop the flame from burning back up into the equipment and causing damage or explosions. The two main types are dry and wet. Each has its own advantages and disadvantages...
. Water is decomposed on-demand into oxyhydrogen, obviating the need for separate hydrogen and oxygen tanks. The original was designed in 1962 by William Rhodes and Raymond Henes of the Henes Manufacturing Co. (now Arizona Hydrogen Manufacturing, Inc.) and marketed under the trade mark "Water Welder". A hypodermic needle
Hypodermic needle
A hypodermic needle is a hollow needle commonly used with a syringe to inject substances into the body or extract fluids from it...
was originally used for the torch tip.
Fringe science and fraud
Oxyhydrogen is sometimes referred to as "Brown's Gas" after Yull Brown who advocated such devices, or "HHO gas" after the claims of fringe physicist Ruggero Santilli.Oxyhydrogen is also often mentioned in conjunction with devices that claim to operate a vehicle using water as a fuel. The most common and decisive counter-argument against producing this gas on-board to use as a fuel or fuel additive is that the energy required to split water molecules exceeds the energy recouped by burning it.
This should also not be confused with hydrogen-fueled cars where the hydrogen is produced elsewhere and used as fuel or where it is used as as fuel enhancement.

